BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting
- PMID: 33626250
- PMCID: PMC7944975
- DOI: 10.1056/NEJMoa2101765
BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting
Abstract
Background: As mass vaccination campaigns against coronavirus disease 2019 (Covid-19) commence worldwide, vaccine effectiveness needs to be assessed for a range of outcomes across diverse populations in a noncontrolled setting. In this study, data from Israel's largest health care organization were used to evaluate the effectiveness of the BNT162b2 mRNA vaccine.
Methods: All persons who were newly vaccinated during the period from December 20, 2020, to February 1, 2021, were matched to unvaccinated controls in a 1:1 ratio according to demographic and clinical characteristics. Study outcomes included documented infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), symptomatic Covid-19, Covid-19-related hospitalization, severe illness, and death. We estimated vaccine effectiveness for each outcome as one minus the risk ratio, using the Kaplan-Meier estimator.
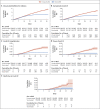
Results: Each study group included 596,618 persons. Estimated vaccine effectiveness for the study outcomes at days 14 through 20 after the first dose and at 7 or more days after the second dose was as follows: for documented infection, 46% (95% confidence interval [CI], 40 to 51) and 92% (95% CI, 88 to 95); for symptomatic Covid-19, 57% (95% CI, 50 to 63) and 94% (95% CI, 87 to 98); for hospitalization, 74% (95% CI, 56 to 86) and 87% (95% CI, 55 to 100); and for severe disease, 62% (95% CI, 39 to 80) and 92% (95% CI, 75 to 100), respectively. Estimated effectiveness in preventing death from Covid-19 was 72% (95% CI, 19 to 100) for days 14 through 20 after the first dose. Estimated effectiveness in specific subpopulations assessed for documented infection and symptomatic Covid-19 was consistent across age groups, with potentially slightly lower effectiveness in persons with multiple coexisting conditions.
Conclusions: This study in a nationwide mass vaccination setting suggests that the BNT162b2 mRNA vaccine is effective for a wide range of Covid-19-related outcomes, a finding consistent with that of the randomized trial.
Copyright © 2021 Massachusetts Medical Society.
Figures
Comment in
-
Blunting COVID-19's negative impact: Lessons from Israel's vaccination campaign.Travel Med Infect Dis. 2021 May-Jun;41:102029. doi: 10.1016/j.tmaid.2021.102029. Epub 2021 Mar 16. Travel Med Infect Dis. 2021. PMID: 33737163 Free PMC article. No abstract available.
-
BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting.N Engl J Med. 2021 May 20;384(20):1968-1970. doi: 10.1056/NEJMc2104281. Epub 2021 Apr 21. N Engl J Med. 2021. PMID: 33882226 No abstract available.
-
Vaccinating People with Obesity for COVID-19: EASO Call for Action.Obes Facts. 2021;14(3):334-335. doi: 10.1159/000516524. Epub 2021 Apr 29. Obes Facts. 2021. PMID: 33915546 Free PMC article. No abstract available.
-
Impact of first dose of BNT162b2 vaccine on COVID-19 infection among healthcare workers in an Irish hospital.Ir J Med Sci. 2022 Apr;191(2):961-962. doi: 10.1007/s11845-021-02658-4. Epub 2021 May 27. Ir J Med Sci. 2022. PMID: 34041693 Free PMC article. No abstract available.
-
Letter from Israel.Respirology. 2022 Jan;27(1):88-89. doi: 10.1111/resp.14180. Epub 2021 Nov 1. Respirology. 2022. PMID: 34725895 No abstract available.
References
-
- ClinicalTrials.gov. Study to describe the safety, tolerability, immunogenicity, and efficacy of RNA vaccine candidates against COVID-19 in healthy individuals. 2020. (https://clinicaltrials.gov/ct2/show/NCT04368728).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
