Ethical and policy implications of vaccinomics in the United States: community members' perspectives
- PMID: 33626296
- PMCID: PMC8189107
- DOI: 10.1080/21645515.2020.1859318
Ethical and policy implications of vaccinomics in the United States: community members' perspectives
Abstract
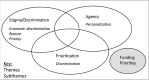
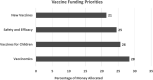
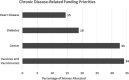
Objectives: We aimed to elucidate public values regarding the use of genomics to improve vaccine development and use (vaccinomics).Methods: Adults ≥18 years-old were recruited through social media and community organizations, and randomly assigned to one of four nested discussion groups in Boulder, CO and Baltimore, MD. Participants rated their confidence in vaccine safety and effectiveness prior to and after discussing vaccinomics. Before departing, they prioritized funding for vaccinomics versus federal priorities (vaccine safety and efficacy, new vaccines, and free vaccines) and chronic diseases (cancer, heart disease, and diabetes). Grounded Theory-influenced methods were used to identify themes.Results: Participants broadly supported vaccinomics. Emergent themes: concerns about reduced privacy/confidentiality, increased genetically based stigma/discrimination, and reduced agency to make vaccine-related decisions through genetically based prioritization. Participants supported vaccinomics' potential for increased personalization. Some participants favored prioritizing others over themselves during a vaccine shortage, while others did not. Some participants worried health insurance companies would discriminate against them based on information discovered through vaccinomics. Participants feared inequitable implementation of vaccinomics would contribute to discrimination and marginalization of vulnerable populations. Discussing vaccinomics did not impact perceptions of vaccine safety and effectiveness. Federal funding for vaccinomics was broadly supported.Conclusion: Participants supported vaccinomics' potential for increased personalization, noting policy safeguards to facilitate equitable implementation and protect privacy were needed. Despite some concerns, participants hoped vaccinomics would improve vaccine safety and effectiveness. Policies regarding vaccinomics' implementation must address public concerns about the privacy and confidentiality of genetic information and potential inequities in access to vaccinomics' benefits.
Keywords: Genomics; Infectious disease; Vacccinomics; Vaccines; Values.
Figures
Similar articles
-
Vaccinomics: a cross-sectional survey of public values.Hum Vaccin Immunother. 2021 Sep 2;17(9):2999-3015. doi: 10.1080/21645515.2021.1911217. Epub 2021 Jun 21. Hum Vaccin Immunother. 2021. PMID: 34152932 Free PMC article.
-
Diagnostic testing for vaccinomics: is the regulatory approval framework adequate? A comparison of Canada, the United States, and Europe.OMICS. 2011 Sep;15(9):597-605. doi: 10.1089/omi.2010.0135. Epub 2011 Jul 5. OMICS. 2011. PMID: 21728814 Review.
-
Vaccinomics: a future avenue for vaccine development against emerging pathogens.Expert Rev Vaccines. 2021 Dec;20(12):1561-1569. doi: 10.1080/14760584.2021.1987222. Epub 2021 Oct 13. Expert Rev Vaccines. 2021. PMID: 34582295 Review.
-
Publics and vaccinomics: beyond public understanding of science.OMICS. 2011 Sep;15(9):607-14. doi: 10.1089/omi.2010.0139. Epub 2011 Jul 6. OMICS. 2011. PMID: 21732820 Review.
-
Vaccinomics and Adversomics in the Era of Precision Medicine: A Review Based on HBV, MMR, HPV, and COVID-19 Vaccines.J Clin Med. 2020 Nov 5;9(11):3561. doi: 10.3390/jcm9113561. J Clin Med. 2020. PMID: 33167413 Free PMC article. Review.
Cited by
-
Vaccinomics: A scoping review.Vaccine. 2023 Mar 31;41(14):2357-2367. doi: 10.1016/j.vaccine.2023.02.009. Epub 2023 Feb 18. Vaccine. 2023. PMID: 36803903 Free PMC article.
-
Vaccinomics: a cross-sectional survey of public values.Hum Vaccin Immunother. 2021 Sep 2;17(9):2999-3015. doi: 10.1080/21645515.2021.1911217. Epub 2021 Jun 21. Hum Vaccin Immunother. 2021. PMID: 34152932 Free PMC article.
-
Nucleotides Entrapped in Liposome Nanovesicles as Tools for Therapeutic and Diagnostic Use in Biomedical Applications.Pharmaceutics. 2023 Mar 8;15(3):873. doi: 10.3390/pharmaceutics15030873. Pharmaceutics. 2023. PMID: 36986734 Free PMC article. Review.
References
-
- Halsey NA, Griffioen M, Dreskin SC, Dekker CL, Wood R, Sharma D, Jones JF, LaRussa PS, Garner J, Berger M, et al. Immediate hypersensitivity reactions following monovalent 2009 pandemic influenza A (H1N1) vaccines: reports to VAERS. Vaccine. 2013;31(51):6107–12. doi:10.1016/j.vaccine.2013.09.066. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical