Salivary SARS-CoV-2 antigen rapid detection: A prospective cohort study
- PMID: 33626369
- PMCID: PMC7897404
- DOI: 10.1016/j.cca.2021.02.014
Salivary SARS-CoV-2 antigen rapid detection: A prospective cohort study
Abstract
Background and aim: SARS-CoV-2 quick testing is relevant for the containment of new pandemic waves. Antigen testing in self-collected saliva might be useful. We compared salivary and naso-pharyngeal swab (NPS) SARS-CoV-2 antigen detection by a rapid chemiluminescent assay (CLEIA) and two different point-of-care (POC) immunochromatographic assays, with results of molecular testing.
Methods: 234 patients were prospectively enrolled. Paired self-collected saliva (Salivette) and NPS were obtained to perform rRT-PCR, chemiluminescent (Lumipulse G) and POC (NPS: Fujirebio and Abbott; saliva: Fujirebio) for SARS-CoV-2 antigen detection.
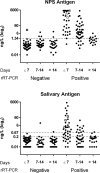
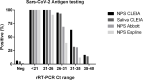
Results: The overall agreement between NPS and saliva rRT-PCR was 78.7%, reaching 91.7% at the first week from symptoms. SARS-CoV-2 CLEIA antigen was highly accurate in distinguishing positive and negative NPS (ROC-AUC = 0.939, 95%CI:0.903-0.977), with 81.6% sensitivity and 93.8% specificity. This assay on saliva reached the optimal value within 7 days from symptoms onset (Sensitivity: 72%; Specificity: 97%). Saliva POC antigen was limited in sensitivity (13%), performing better in NPS (Sensitivity: 48% and 66%; Specificity: 100% and 99% for Espline and Abbott respectively), depending on viral loads.
Conclusions: Self-collected saliva is a valid alternative to NPS for SARS-CoV-2 detection by molecular, but also by CLEIA antigen testing, which is therefore potentially useful for large scale screening.
Keywords: COVID-19; Chemiluminescence; Naso-pharyngeal swab; Point-of-care.
Copyright © 2021 Elsevier B.V. All rights reserved.
Figures

References
-
- D. Sapkota, T.M. Søland, H.K. Galtung, L.P. Sand, S. Giannecchini, K.K.W. To, et al., COVID-19 salivary signature: diagnostic and research opportunities, J. Clin. Pathol. (2020). https://doi:10.1136/jclinpath-2020-206834. - PubMed
-
- Migueres M., Mengelle C., Dimeglio C., Didier A., Alvarez M., Delobel P., Mansuy J.-M., Izopet J. Saliva sampling for diagnosing SARS-CoV-2 infections in symptomatic patients and asymptomatic carriers. Journal of Clinical Virology. 2020;130:104580. doi: 10.1016/j.jcv.2020.104580. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

