Dual cytoplasmic and nuclear localization of HTLV-1-encoded HBZ protein is a unique feature of adult T-cell leukemia
- PMID: 33626865
- PMCID: PMC8327710
- DOI: 10.3324/haematol.2020.272468
Dual cytoplasmic and nuclear localization of HTLV-1-encoded HBZ protein is a unique feature of adult T-cell leukemia
Abstract
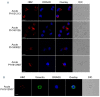
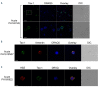
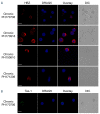
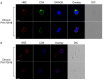
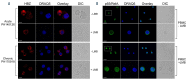
Adult T-cell leukemia-lymphoma (ATL), is a highly malignant T-cell neoplasm caused by human T-cell leukemia virus type 1 (HTLV-1), characterized by a poor prognosis. Two viral proteins, Tax-1 and HBZ play important roles in the pathogenesis of ATL. While Tax-1 can be found in both cytoplasm and nucleus of HTLV-1 infected patients, HBZ is exclusively localized in the cytoplasm of HTLV-1 asymptomatic carriers and patients with chronic neurologic disease HAM/TSP, and only in the nucleus of ATL cell lines, suggesting that the nuclear localization of HBZ can be a hallmark of neoplastic transformation. To clarify this crucial point, here we investigated in detail the pattern of HBZ expression in ATL patients. We made use of our monoclonal antibody 4D4-F3, that at present is a uniquely reported reagent, among the few described, able to detect endogenous HBZ by immunofluorescence and confocal microscopy in cells from asymptomatic carriers, HAM/TSP and ATL patients. We found that HBZ localizes both in the cytoplasm and in the nucleus of cells of ATL patients irrespective of their clinical status, with a strong preference for the cytoplasmic localization. Also Tax-1 localized in both compartments. As HBZ is exclusively localized in the cytoplasm in asymptomatic carriers and in non-neoplastic pathologies, this finding shows that neoplastic transformation consequent to HTLV-1 infection is accompanied and associated with the capacity of HBZ to translocate to the nucleus, which suggests a role of cytoplasmic-to-nuclear translocation in HTLV-1-mediated oncogenesis.
Figures
Comment in
-
Is it NICE (nuclear import as a carcinogenic mechanism) to restrict HBZ in the cytoplasm?Haematologica. 2021 Aug 1;106(8):2038. doi: 10.3324/haematol.2021.278377. Haematologica. 2021. PMID: 33626868 Free PMC article. No abstract available.
Similar articles
-
Cytoplasmic Localization of HTLV-1 HBZ Protein: A Biomarker of HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis (HAM/TSP).PLoS Negl Trop Dis. 2017 Jan 17;11(1):e0005285. doi: 10.1371/journal.pntd.0005285. eCollection 2017 Jan. PLoS Negl Trop Dis. 2017. PMID: 28095504 Free PMC article.
-
Localization, quantification and interaction with host factors of endogenous HTLV-1 HBZ protein in infected cells and ATL.Retrovirology. 2015 Jul 4;12:59. doi: 10.1186/s12977-015-0186-0. Retrovirology. 2015. PMID: 26140924 Free PMC article.
-
In vivo expression of the HBZ gene of HTLV-1 correlates with proviral load, inflammatory markers and disease severity in HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP).Retrovirology. 2009 Feb 19;6:19. doi: 10.1186/1742-4690-6-19. Retrovirology. 2009. PMID: 19228429 Free PMC article.
-
Adult T-cell leukemia-lymphoma as a viral disease: Subtypes based on viral aspects.Cancer Sci. 2021 May;112(5):1688-1694. doi: 10.1111/cas.14869. Epub 2021 Mar 30. Cancer Sci. 2021. PMID: 33630351 Free PMC article. Review.
-
HTLV-1 Infection and Adult T-Cell Leukemia/Lymphoma-A Tale of Two Proteins: Tax and HBZ.Viruses. 2016 Jun 16;8(6):161. doi: 10.3390/v8060161. Viruses. 2016. PMID: 27322308 Free PMC article. Review.
Cited by
-
The Road to HTLV-1-Induced Leukemia by Following the Subcellular Localization of HTLV-1-Encoded HBZ Protein.Front Immunol. 2022 Jun 23;13:940131. doi: 10.3389/fimmu.2022.940131. eCollection 2022. Front Immunol. 2022. PMID: 35812456 Free PMC article. Review.
-
HTLV-1 Infection and Pathogenesis: New Insights from Cellular and Animal Models.Int J Mol Sci. 2021 Jul 27;22(15):8001. doi: 10.3390/ijms22158001. Int J Mol Sci. 2021. PMID: 34360767 Free PMC article. Review.
-
JunB-HBZ nuclear translocation by TGF-β is a key driver in HTLV-1-mediated leukemogenesis.Proc Natl Acad Sci U S A. 2025 Jul;122(26):e2420756122. doi: 10.1073/pnas.2420756122. Epub 2025 Jun 23. Proc Natl Acad Sci U S A. 2025. PMID: 40549917 Free PMC article.
-
Viral oncogenesis in cancer: from mechanisms to therapeutics.Signal Transduct Target Ther. 2025 May 12;10(1):151. doi: 10.1038/s41392-025-02197-9. Signal Transduct Target Ther. 2025. PMID: 40350456 Free PMC article. Review.
-
YTHDF1 and YTHDC1 m6A reader proteins regulate HTLV-1 tax and hbz activity.J Virol. 2025 Mar 18;99(3):e0206324. doi: 10.1128/jvi.02063-24. Epub 2025 Feb 4. J Virol. 2025. PMID: 39902961 Free PMC article.
References
-
- Gessain A, Vernant JC, Maurs L, et al. . Antibodies to human T-lymphotropic virus type-1 in patients with tropical spastic paraparesis Lancet. 1985;326(8452):407-410. - PubMed
-
- Osame M, Usuku K, Izumo S, et al. . HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;327(8488):1031-1032. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

