Transcription Factor MAFF (MAF Basic Leucine Zipper Transcription Factor F) Regulates an Atherosclerosis Relevant Network Connecting Inflammation and Cholesterol Metabolism
- PMID: 33626882
- PMCID: PMC8124091
- DOI: 10.1161/CIRCULATIONAHA.120.050186
Transcription Factor MAFF (MAF Basic Leucine Zipper Transcription Factor F) Regulates an Atherosclerosis Relevant Network Connecting Inflammation and Cholesterol Metabolism
Abstract
Background: Coronary artery disease (CAD) is a multifactorial condition with both genetic and exogenous causes. The contribution of tissue-specific functional networks to the development of atherosclerosis remains largely unclear. The aim of this study was to identify and characterize central regulators and networks leading to atherosclerosis.
Methods: Based on several hundred genes known to affect atherosclerosis risk in mouse (as demonstrated in knockout models) and human (as shown by genome-wide association studies), liver gene regulatory networks were modeled. The hierarchical order and regulatory directions of genes within the network were based on Bayesian prediction models, as well as experimental studies including chromatin immunoprecipitation DNA-sequencing, chromatin immunoprecipitation mass spectrometry, overexpression, small interfering RNA knockdown in mouse and human liver cells, and knockout mouse experiments. Bioinformatics and correlation analyses were used to clarify associations between central genes and CAD phenotypes in both human and mouse.
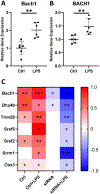
Results: The transcription factor MAFF (MAF basic leucine zipper transcription factor F) interacted as a key driver of a liver network with 3 human genes at CAD genome-wide association studies loci and 11 atherosclerotic murine genes. Most importantly, expression levels of the low-density lipoprotein receptor (LDLR) gene correlated with MAFF in 600 CAD patients undergoing bypass surgery (STARNET [Stockholm-Tartu Atherosclerosis Reverse Network Engineering Task]) and a hybrid mouse diversity panel involving 105 different inbred mouse strains. Molecular mechanisms of MAFF were tested in noninflammatory conditions and showed positive correlation between MAFF and LDLR in vitro and in vivo. Interestingly, after lipopolysaccharide stimulation (inflammatory conditions), an inverse correlation between MAFF and LDLR in vitro and in vivo was observed. Chromatin immunoprecipitation mass spectrometry revealed that the human CAD genome-wide association studies candidate BACH1 (BTB domain and CNC homolog 1) assists MAFF in the presence of lipopolysaccharide stimulation with respective heterodimers binding at the MAF recognition element of the LDLR promoter to transcriptionally downregulate LDLR expression.
Conclusions: The transcription factor MAFF was identified as a novel central regulator of an atherosclerosis/CAD-relevant liver network. MAFF triggered context-specific expression of LDLR and other genes known to affect CAD risk. Our results suggest that MAFF is a missing link between inflammation, lipid and lipoprotein metabolism, and a possible treatment target.
Keywords: atherosclerosis; chromatin immunoprecipitation; coronary artery disease; inflammation; lipopolysaccharides; mafF transcription factor; receptors, LDL.
Figures







References
-
- Writing Group M, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP et al. Executive Summary: Heart Disease and Stroke Statistics--2016 Update: A Report From the American Heart Association. Circulation. 2016;133:447–54. - PubMed
-
- Nichols M, Townsend N, Scarborough P and Rayner M. Cardiovascular disease in Europe 2014: epidemiological update. Eur Heart J. 2014;35:2929. - PubMed
MeSH terms
Substances
Grants and funding
- R01 HL144651/HL/NHLBI NIH HHS/United States
- R00 HL138193/HL/NHLBI NIH HHS/United States
- R01 DK117850/DK/NIDDK NIH HHS/United States
- P01 HL028481/HL/NHLBI NIH HHS/United States
- R01 HL147883/HL/NHLBI NIH HHS/United States
- K08 HL111330/HL/NHLBI NIH HHS/United States
- K23 HL111339/HL/NHLBI NIH HHS/United States
- R01 DK102559/DK/NIDDK NIH HHS/United States
- R01 HL125863/HL/NHLBI NIH HHS/United States
- R01 AG050986/AG/NIA NIH HHS/United States
- R01 HL135093/HL/NHLBI NIH HHS/United States
- R01 HL071207/HL/NHLBI NIH HHS/United States
- R01 DK104363/DK/NIDDK NIH HHS/United States
- P01 HL030568/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

