Short-chain fatty acids contribute to neuropathic pain via regulating microglia activation and polarization
- PMID: 33626986
- PMCID: PMC7925956
- DOI: 10.1177/1744806921996520
Short-chain fatty acids contribute to neuropathic pain via regulating microglia activation and polarization
Abstract
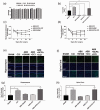
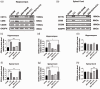
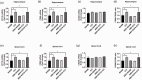
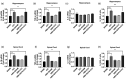
Microglia activation and subsequent pro-inflammatory responses play a key role in the development of neuropathic pain. The process of microglia polarization towards pro-inflammatory phenotype often occurs during neuroinflammation. Recent studies have demonstrated an active role for the gut microbiota in promoting microglial full maturation and inflammatory capabilities via the production of Short-Chain Fatty Acids (SCFAs). However, it remains unclear whether SCFAs is involved in pro-inflammatory/anti-inflammatory phenotypes microglia polarization in the neuropathic pain. In the present study, chronic constriction injury (CCI) was used to induce neuropathic pain in mice, the mechanical withdrawal threshold, thermal hyperalgesia were accomplished. The levels of microglia markers including ionized calcium-binding adaptor molecule 1 (Iba1), cluster of differentiation 11b (CD11b), pro-inflammatory phenotype markers including CD68, interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and anti-inflammatory phenotype markers including CD206, IL-4 in the hippocampus and spinal cord were determined on day 21 after CCI. The results showed that CCI produced mechanical allodynia and thermal hyperalgesia, and also increased the expressions of microglia markers (Iba1, CD11b) and pro-inflammatory phenotype markers (CD68, IL-1β, and TNF-α), but not anti-inflammatory phenotype marker (CD206, IL-4) in the hippocampus and spinal cord, accompanied by increased SCFAs in the gut. Notably, antibiotic administration reversed these abnormalities, and its effects was also bloked by SCFAs administration. In conclusion, data from our study suggest that CCI can lead to mechanical and thermal hyperalgesia, while SCFAs play a key role in the pathogenesis of neuropathic pain by regulating microglial activation and subsequent pro-inflammatory phenotype polarization. Antibiotic administration may be a new treatment for neuropathic pain by reducing the production of SCFAs and further inhibiting the process of microglia polarization.
Keywords: SCFAs; gut microbiota; microglial polarization; neuropathic pain.
Conflict of interest statement
Figures


Similar articles
-
Spared Nerve Injury Increases the Expression of Microglia M1 Markers in the Prefrontal Cortex of Rats and Provokes Depression-Like Behaviors.Front Neurosci. 2017 Apr 18;11:209. doi: 10.3389/fnins.2017.00209. eCollection 2017. Front Neurosci. 2017. PMID: 28458629 Free PMC article.
-
Anti-inflammatory protein TSG-6 secreted by bone marrow mesenchymal stem cells attenuates neuropathic pain by inhibiting the TLR2/MyD88/NF-κB signaling pathway in spinal microglia.J Neuroinflammation. 2020 May 11;17(1):154. doi: 10.1186/s12974-020-1731-x. J Neuroinflammation. 2020. PMID: 32393298 Free PMC article.
-
Administration of 2-deoxy-D-glucose alleviates cancer-induced bone pain by suppressing microglial polarization to the M1 phenotype and neuroinflammation.Mol Pain. 2025 Jan-Dec;21:17448069251348778. doi: 10.1177/17448069251348778. Epub 2025 May 30. Mol Pain. 2025. PMID: 40444883 Free PMC article.
-
Immune phenotypes of microglia in human neurodegenerative disease: challenges to detecting microglial polarization in human brains.Alzheimers Res Ther. 2015 Aug 19;7(1):56. doi: 10.1186/s13195-015-0139-9. Alzheimers Res Ther. 2015. PMID: 26286145 Free PMC article. Review.
-
The Role of TNF-α in Neuropathic Pain: An Immunotherapeutic Perspective.Life (Basel). 2025 May 14;15(5):785. doi: 10.3390/life15050785. Life (Basel). 2025. PMID: 40430212 Free PMC article. Review.
Cited by
-
Imbalance of Microbacterial Diversity Is Associated with Functional Prognosis of Stroke.Neural Plast. 2023 May 8;2023:6297653. doi: 10.1155/2023/6297653. eCollection 2023. Neural Plast. 2023. PMID: 37197229 Free PMC article.
-
The gut microbiota-neuroimmune crosstalk and neuropathic pain: a scoping review.Gut Microbiome (Camb). 2023 Jun 19;4:e10. doi: 10.1017/gmb.2023.7. eCollection 2023. Gut Microbiome (Camb). 2023. PMID: 39295900 Free PMC article.
-
STING Contributes to Cancer-Induced Bone Pain by Promoting M1 Polarization of Microglia in the Medial Prefrontal Cortex.Cancers (Basel). 2022 Oct 22;14(21):5188. doi: 10.3390/cancers14215188. Cancers (Basel). 2022. PMID: 36358605 Free PMC article.
-
Neuro-Immunity and Gut Dysbiosis Drive Parkinson's Disease-Induced Pain.Front Immunol. 2021 Nov 18;12:759679. doi: 10.3389/fimmu.2021.759679. eCollection 2021. Front Immunol. 2021. PMID: 34868000 Free PMC article. Review.
-
Relationship Between Short-chain Fatty Acids and Parkinson's Disease: A Review from Pathology to Clinic.Neurosci Bull. 2024 Apr;40(4):500-516. doi: 10.1007/s12264-023-01123-9. Epub 2023 Sep 27. Neurosci Bull. 2024. PMID: 37755674 Free PMC article. Review.
References
-
- O'Connor A. Neuropathic pain: quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics 2009; 27: 95–112. - PubMed
-
- Gilron I, Baron R, Jensen T. Neuropathic pain: principles of diagnosis and treatment. Mayo Clin Proc 2015; 90: 532–545. - PubMed
-
- Sra A, Smith P. Etiology and pharmacology of neuropathic pain. Pharmacol Rev 2018; 70: 315–347. - PubMed
-
- Guo R, Chen L, Xing C, et al. Pain regulation by gut microbiota: molecular mechanisms and therapeutic potential. Br J Anaesth 2019; 123: 637–654. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

