Effect of screening by clinical breast examination on breast cancer incidence and mortality after 20 years: prospective, cluster randomised controlled trial in Mumbai
- PMID: 33627312
- PMCID: PMC7903383
- DOI: 10.1136/bmj.n256
Effect of screening by clinical breast examination on breast cancer incidence and mortality after 20 years: prospective, cluster randomised controlled trial in Mumbai
Erratum in
-
Effect of screening by clinical breast examination on breast cancer incidence and mortality after 20 years: prospective, cluster randomised controlled trial in Mumbai.BMJ. 2021 Mar 19;372:n738. doi: 10.1136/bmj.n738. BMJ. 2021. PMID: 33741550 Free PMC article. No abstract available.
Abstract
Objective: To test the efficacy of screening by clinical breast examination in downstaging breast cancer at diagnosis and in reducing mortality from the disease, when compared with no screening.
Design: Prospective, cluster randomised controlled trial.
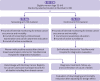
Setting: 20 geographically distinct clusters located in Mumbai, India, randomly allocated to 10 screening and 10 control clusters; total trial duration was 20 years (recruitment began in May 1998; database locked in March 2019 for analysis).
Participants: 151 538 women aged 35-64 with no history of breast cancer.
Interventions: Women in the screening arm (n=75 360) received four screening rounds of clinical breast examination (conducted by trained female primary health workers) and cancer awareness every two years, followed by five rounds of active surveillance every two years. Women in the control arm (n=76 178) received one round of cancer awareness followed by eight rounds of active surveillance every two years.
Main outcome measures: Downstaging of breast cancer at diagnosis and reduction in mortality from breast cancer.
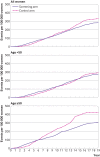
Results: Breast cancer was detected at an earlier age in the screening group than in the control group (age 55.18 (standard deviation 9.10) v 56.50 (9.10); P=0.01), with a significant reduction in the proportion of women with stage III or IV disease (37% (n=220) v 47% (n=271), P=0.001). A non-significant 15% reduction in breast cancer mortality was observed in the screening arm versus control arm in the overall study population (age 35-64; 20.82 deaths per 100 000 person years (95% confidence interval 18.25 to 23.97) v 24.62 (21.71 to 28.04); rate ratio 0.85 (95% confidence interval 0.71 to 1.01); P=0.07). However, a post hoc subset analysis showed nearly 30% relative reduction in breast cancer mortality in women aged 50 and older (24.62 (20.62 to 29.76) v 34.68 (27.54 to 44.37); 0.71 (0.54 to 0.94); P=0.02), but no significant reduction in women younger than 50 (19.53 (17.24 to 22.29) v 21.03 (18.97 to 23.44); 0.93 (0.79 to 1.09); P=0.37). A 5% reduction in all cause mortality was seen in the screening arm versus the control arm, but it was not statistically significant (rate ratio 0.95 (95% confidence interval 0.81 to 1.10); P=0.49).
Conclusions: These results indicate that clinical breast examination conducted every two years by primary health workers significantly downstaged breast cancer at diagnosis and led to a non-significant 15% reduction in breast cancer mortality overall (but a significant reduction of nearly 30%in mortality in women aged ≥50). No significant reduction in mortality was seen in women younger than 50 years. Clinical breast examination should be considered for breast cancer screening in low and middle income countries.
Trial registration: Clinical Trials Registry of India CTRI/2010/091/001205; ClinicalTrials.gov NCT00632047.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the US National Institutes of Health, Tata Memorial Centre, Sir Dorabji Tata Trusts, M K Tata Trusts, Department of Atomic Energy Clinical Trial Centre (Government of India), and the Women’s Cancer Initiative, India for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures
Comment in
-
Self-breast examination for breast cancer screening: The Indian story.Indian J Cancer. 2022 Jan-Mar;59(1):1-3. doi: 10.4103/ijc.ijc_447_22. Indian J Cancer. 2022. PMID: 35645048 No abstract available.
Similar articles
-
Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial.BMJ. 2014 Feb 11;348:g366. doi: 10.1136/bmj.g366. BMJ. 2014. PMID: 24519768 Free PMC article. Clinical Trial.
-
Evaluation of mammographic surveillance services in women aged 40-49 years with a moderate family history of breast cancer: a single-arm cohort study.Health Technol Assess. 2013 Mar;17(11):vii-xiv, 1-95. doi: 10.3310/hta17110. Health Technol Assess. 2013. PMID: 23489892 Free PMC article. Clinical Trial.
-
Effectiveness of triennial screening with clinical breast examination: 14-years follow-up outcomes of randomized clinical trial in Trivandrum, India.Cancer. 2023 Jan 15;129(2):272-282. doi: 10.1002/cncr.34526. Epub 2022 Nov 1. Cancer. 2023. PMID: 36321193 Free PMC article. Clinical Trial.
-
Long-term effects of mammography screening: updated overview of the Swedish randomised trials.Lancet. 2002 Mar 16;359(9310):909-19. doi: 10.1016/S0140-6736(02)08020-0. Lancet. 2002. PMID: 11918907 Review.
-
Improving early detection of breast cancer in sub-Saharan Africa: why mammography may not be the way forward.Global Health. 2019 Jan 8;15(1):3. doi: 10.1186/s12992-018-0446-6. Global Health. 2019. PMID: 30621753 Free PMC article. Review.
Cited by
-
Breast cancer screening patterns and associated factors in Iranian women over 40 years.Sci Rep. 2024 Jul 3;14(1):15274. doi: 10.1038/s41598-024-66342-0. Sci Rep. 2024. PMID: 38961238 Free PMC article.
-
Management of BRCA-associated breast cancer patients in low and middle-income countries: a review.Ecancermedicalscience. 2024 Aug 22;18:1744. doi: 10.3332/ecancer.2024.1744. eCollection 2024. Ecancermedicalscience. 2024. PMID: 39421188 Free PMC article. Review.
-
Research studies on screening tests.Perspect Clin Res. 2022 Jul-Sep;13(3):168-171. doi: 10.4103/picr.picr_111_22. Epub 2022 Jun 30. Perspect Clin Res. 2022. PMID: 35928637 Free PMC article.
-
Breast Cancer Screening in Semi-Rural Malaysia: Utilisation and Barriers.Int J Environ Res Public Health. 2021 Nov 23;18(23):12293. doi: 10.3390/ijerph182312293. Int J Environ Res Public Health. 2021. PMID: 34886015 Free PMC article.
-
A real world evaluation of an innovative artificial intelligence tool for population-level breast cancer screening.NPJ Digit Med. 2025 Jan 2;8(1):2. doi: 10.1038/s41746-024-01368-2. NPJ Digit Med. 2025. PMID: 39748126 Free PMC article.
References
-
- International Agency for Research on Cancer. CI5 plus-cancer incidence in five continents time trends. 2018. https://ci5.iarc.fr/CI5plus/default.aspx
-
- Mumbai Cancer Registry . Annual reports 1992-2016: cancer incidence and mortality in Mumbai Municipal Corporation area. Mumbai. Indian Cancer Society, 2016.
-
- Dhillon PK, Mathur P, Nandakumar A, India State-Level Disease Burden Initiative Cancer Collaborators . The burden of cancers and their variations across the states of India: the Global Burden of Disease Study 1990-2016. Lancet Oncol 2018;19:1289-306. 10.1016/S1470-2045(18)30447-9 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical