Hypotension in Preterm Infants (HIP) randomised trial
- PMID: 33627329
- PMCID: PMC8237176
- DOI: 10.1136/archdischild-2020-320241
Hypotension in Preterm Infants (HIP) randomised trial
Abstract
Objective: To determine whether restricting the use of inotrope after diagnosis of low blood pressure (BP) in the first 72 hours of life affects survival without significant brain injury at 36 weeks of postmenstrual age (PMA) in infants born before 28 weeks of gestation.
Design: Double-blind, placebo-controlled randomised trial. Caregivers were masked to group assignment.
Setting: 10 sites across Europe and Canada.
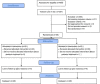
Participants: Infants born before 28 weeks of gestation were eligible if they had an invasive mean BP less than their gestational age that persisted for ≥15 min in the first 72 hours of life and a cerebral ultrasound free of significant (≥ grade 3) intraventricular haemorrhage.
Intervention: Participants were randomly assigned to saline bolus followed by either a dopamine infusion (standard management) or placebo (5% dextrose) infusion (restrictive management).
Primary outcome: Survival to 36 weeks of PMA without severe brain injury.
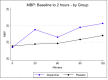
Results: The trial terminated early due to significant enrolment issues (7.7% of planned recruitment). 58 infants were enrolled between February 2015 and September 2017. The two groups were well matched for baseline variables. In the standard group, 18/29 (62%) achieved the primary outcome compared with 20/29 (69%) in the restrictive group (p=0.58). Additional treatments for low BP were used less frequently in the standard arm (11/29 (38%) vs 19/29 (66%), p=0.038).
Conclusion: Though this study lacked power, we did not detect major differences in clinical outcomes between standard or restrictive approach to treatment. These results will inform future studies in this area.
Trial registration number: NCT01482559, EudraCT 2010-023988-17.
Keywords: cardiology; neonatology; pharmacology.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Comment in
-
When perfection is the enemy of good: EBM lessons from the HIP trial.J Perinatol. 2023 Feb;43(2):253-256. doi: 10.1038/s41372-022-01554-8. Epub 2022 Nov 14. J Perinatol. 2023. PMID: 36376449 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical