Effect of an Adenovirus-Vectored Universal Influenza Virus Vaccine on Pulmonary Pathophysiology in a Mouse Model
- PMID: 33627390
- PMCID: PMC8104105
- DOI: 10.1128/JVI.02359-20
Effect of an Adenovirus-Vectored Universal Influenza Virus Vaccine on Pulmonary Pathophysiology in a Mouse Model
Abstract
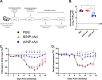
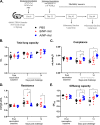
Current influenza vaccines, live attenuated or inactivated, do not protect against antigenically novel influenza A viruses (IAVs) of pandemic potential, which has driven interest in the development of universal influenza vaccines. Universal influenza vaccine candidates targeting highly conserved antigens of IAV nucleoprotein (NP) are promising as vaccines that induce T cell immunity, but concerns have been raised about the safety of inducing robust CD8 T cell responses in the lungs. Using a mouse model, we systematically evaluated effects of recombinant adenovirus vectors (rAd) expressing IAV NP (A/NP-rAd) or influenza B virus (IBV) NP (B/NP-rAd) on pulmonary inflammation and function after vaccination and following live IAV challenge. After A/NP-rAd or B/NP-rAd vaccination, female mice exhibited robust systemic and pulmonary vaccine-specific B cell and T cell responses and experienced no morbidity (e.g., body mass loss). Both in vivo pulmonary function testing and lung histopathology scoring revealed minimal adverse effects of intranasal rAd vaccination compared with unvaccinated mice. After IAV challenge, A/NP-rAd-vaccinated mice experienced significantly less morbidity, had lower pulmonary virus titers, and developed less pulmonary inflammation than unvaccinated or B/NP-rAd-vaccinated mice. Based on analysis of pulmonary physiology using detailed testing not previously applied to the question of T cell damage, mice protected by vaccination also had better lung function than controls. Results provide evidence that, in this model, adenoviral universal influenza vaccine does not damage pulmonary tissue. In addition, adaptive immunity, in particular, T cell immunity in the lungs, does not cause damage when restimulated but instead mitigates pulmonary damage following IAV infection.IMPORTANCE Respiratory viruses can emerge and spread rapidly before vaccines are available. It would be a tremendous advance to use vaccines that protect against whole categories of viruses, such as universal influenza vaccines, without the need to predict which virus will emerge. The nucleoprotein (NP) of influenza virus provides a target conserved among strains and is a dominant T cell target. In animals, vaccination to NP generates powerful T cell immunity and long-lasting protection against diverse influenza strains. Concerns have been raised, but not evaluated experimentally, that potent local T cell responses might damage the lungs. We analyzed lung function in detail in the setting of such a vaccination. Despite CD8 T cell responses in the lungs, lungs were not damaged and functioned normally after vaccination alone and were protected upon subsequent infection. This precedent provides important support for vaccines based on T cell-mediated protection, currently being considered for both influenza and SARS-CoV-2 vaccines.
Keywords: T cell response; adenovirus; antibody; influenza A virus; influenza B virus; oxygen exchange; pulmonary function; recombinant adenovirus; universal influenza vaccine; vaccine safety.
Copyright © 2021 American Society for Microbiology.
Figures



References
-
- Kallewaard NL, Corti D, Collins PJ, Neu U, McAuliffe JM, Benjamin E, Wachter-Rosati L, Palmer-Hill FJ, Yuan AQ, Walker PA, Vorlaender MK, Bianchi S, Guarino B, De Marco A, Vanzetta F, Agatic G, Foglierini M, Pinna D, Fernandez-Rodriguez B, Fruehwirth A, Silacci C, Ogrodowicz RW, Martin SR, Sallusto F, Suzich JA, Lanzavecchia A, Zhu Q, Gamblin SJ, Skehel JJ. 2016. Structure and function analysis of an antibody recognizing all influenza A subtypes. Cell 166:596–608. 10.1016/j.cell.2016.05.073. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

