Generating tumor-selective conditionally active biologic anti-CTLA4 antibodies via protein-associated chemical switches
- PMID: 33627407
- PMCID: PMC7936328
- DOI: 10.1073/pnas.2020606118
Generating tumor-selective conditionally active biologic anti-CTLA4 antibodies via protein-associated chemical switches
Abstract
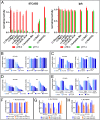
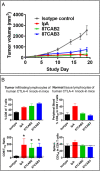
Anticytotoxic T lymphocyte-associated protein 4 (CTLA4) antibodies have shown potent antitumor activity, but systemic immune activation leads to severe immune-related adverse events, limiting clinical usage. We developed novel, conditionally active biologic (CAB) anti-CTLA4 antibodies that are active only in the acidic tumor microenvironment. In healthy tissue, this binding is reversibly inhibited by a novel mechanism using physiological chemicals as protein-associated chemical switches (PaCS). No enzymes or potentially immunogenic covalent modifications to the antibody are required for activation in the tumor. The novel anti-CTLA4 antibodies show similar efficacy in animal models compared to an analog of a marketed anti-CTLA4 biologic, but have markedly reduced toxicity in nonhuman primates (in combination with an anti-PD1 checkpoint inhibitor), indicating a widened therapeutic index (TI). The PaCS encompass mechanisms that are applicable to a wide array of antibody formats (e.g., ADC, bispecifics) and antigens. Examples shown here include antibodies to EpCAM, Her2, Nectin4, CD73, and CD3. Existing antibodies can be engineered readily to be made sensitive to PaCS, and the inhibitory activity can be optimized for each antigen's varying expression level and tissue distribution. PaCS can modulate diverse physiological molecular interactions and are applicable to various pathologic conditions, enabling differential CAB antibody activities in normal versus disease microenvironments.
Keywords: CTLA4; conditionally active biologics; immunooncology; monoclonal antibodies; protein-associated chemical switches.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
Competing interest statement: All authors are shareholders of BioAtla, Inc., which owns the intellectual property rights to CABs- and PaCS-related technologies. H.W.C., G.F., and J.M.S. are inventors on relevant patents. (L.S. is not an inventor on relevant patents.) L.S. and J.M.S. serve as Directors of BioAtla.
Figures


References
-
- Junghans R. P., The challenges of solid tumor for designer CAR-T therapies: A 25-year perspective. Cancer Gene Ther. 24, 89–99 (2017). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

