Rescuing the Last-Line Polymyxins: Achievements and Challenges
- PMID: 33627412
- PMCID: PMC7911091
- DOI: 10.1124/pharmrev.120.000020
Rescuing the Last-Line Polymyxins: Achievements and Challenges
Abstract
Antibiotic resistance is a major global health challenge and, worryingly, several key Gram negative pathogens can become resistant to most currently available antibiotics. Polymyxins have been revived as a last-line therapeutic option for the treatment of infections caused by multidrug-resistant Gram negative bacteria, in particular Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacterales. Polymyxins were first discovered in the late 1940s but were abandoned soon after their approval in the late 1950s as a result of toxicities (e.g., nephrotoxicity) and the availability of "safer" antibiotics approved at that time. Therefore, knowledge on polymyxins had been scarce until recently, when enormous efforts have been made by several research teams around the world to elucidate the chemical, microbiological, pharmacokinetic/pharmacodynamic, and toxicological properties of polymyxins. One of the major achievements is the development of the first scientifically based dosage regimens for colistin that are crucial to ensure its safe and effective use in patients. Although the guideline has not been developed for polymyxin B, a large clinical trial is currently being conducted to optimize its clinical use. Importantly, several novel, safer polymyxin-like lipopeptides are developed to overcome the nephrotoxicity, poor efficacy against pulmonary infections, and narrow therapeutic windows of the currently used polymyxin B and colistin. This review discusses the latest achievements on polymyxins and highlights the major challenges ahead in optimizing their clinical use and discovering new-generation polymyxins. To save lives from the deadly infections caused by Gram negative "superbugs," every effort must be made to improve the clinical utility of the last-line polymyxins. SIGNIFICANCE STATEMENT: Antimicrobial resistance poses a significant threat to global health. The increasing prevalence of multidrug-resistant (MDR) bacterial infections has been highlighted by leading global health organizations and authorities. Polymyxins are a last-line defense against difficult-to-treat MDR Gram negative pathogens. Unfortunately, the pharmacological information on polymyxins was very limited until recently. This review provides a comprehensive overview on the major achievements and challenges in polymyxin pharmacology and clinical use and how the recent findings have been employed to improve clinical practice worldwide.
Copyright © 2021 by The Author(s).
Conflict of interest statement
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health. J.L. is an Australia National Health and Medical Research Council (NHMRC) Principal Research Fellow. The novel polymyxin candidates developed by J.L. and T.V.’s group were licensed to Qpex Biopharma. J.L. received seminar honoraria on the pharmacology of colistin and polymyxin B from Genentech, Inc.; DocMode; Healcare Pharmaceuticals; and a number of international conferences and research grants from Northern Antibiotics. No author has other actual or perceived conflicts of interest with the other contents of this article.
Figures
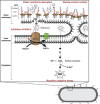
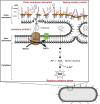
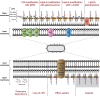
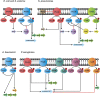
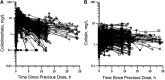


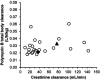
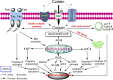
References
-
- Abdul Rahim N, Cheah SE, Johnson MD, Zhu Y, Yu HH, Sidjabat HE, Butler MS, Cooper MA, Fu J, Paterson DL, et al. (2020) Transcriptomic responses of a New Delhi metallo-β-lactamase-producing Klebsiella pneumoniae isolate to the combination of polymyxin B and chloramphenicol. Int J Antimicrob Agents 56:106061. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

