Atmospheric aging enhances the ice nucleation ability of biomass-burning aerosol
- PMID: 33627419
- PMCID: PMC7904250
- DOI: 10.1126/sciadv.abd3440
Atmospheric aging enhances the ice nucleation ability of biomass-burning aerosol
Abstract
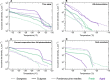
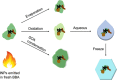
Ice-nucleating particles (INPs) in biomass-burning aerosol (BBA) that affect cloud glaciation, microphysics, precipitation, and radiative forcing were recently found to be driven by the production of mineral phases. BBA experiences extensive chemical aging as the smoke plume dilutes, and we explored how this alters the ice activity of the smoke using simulated atmospheric aging of authentic BBA in a chamber reactor. Unexpectedly, atmospheric aging enhanced the ice activity for most types of fuels and aging schemes. The removal of organic carbon particle coatings that conceal the mineral-based ice-active sites by evaporation or oxidation then dissolution can increase the ice activity by greater than an order of magnitude. This represents a different framework for the evolution of INPs from biomass burning where BBA becomes more ice active as it dilutes and ages, making a larger contribution to the INP budget, resulting cloud microphysics, and climate forcing than is currently considered.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures


References
-
- Fromm M., Peterson D., Di Girolamo L., The primary convective pathway for observed wildfire emissions in the upper troposphere and lower stratosphere: A targeted reinterpretation. J. Geophys. Res. Atmos. 124, 13254–13272 (2019).
-
- Stevens-Rumann C. S., Kemp K. B., Higuera P. E., Harvey B. J., Rother M. T., Donato D. C., Morgan P., Veblen T. T., Evidence for declining forest resilience to wildfires under climate change. Ecol. Lett. 21, 243–252 (2018). - PubMed
-
- O’Dell K., Ford B., Fischer E. V., Pierce J. R., Contribution of wildland-fire smoke to US PM2.5 and its influence on recent trends. Environ. Sci. Technol. 53, 1797–1804 (2019). - PubMed
-
- Williams A. P., Abatzoglou J. T., Gershunov A., Guzman-Morales J., Bishop D. A., Balch J. K., Lettenmaier D. P., Observed impacts of anthropogenic climate change on wildfire in California. Earth’s Future 7, 892–910 (2019).
LinkOut - more resources
Full Text Sources
Other Literature Sources

