Association of Memory Impairment With Concomitant Tau Pathology in Patients With Cerebral Amyloid Angiopathy
- PMID: 33627498
- PMCID: PMC8166424
- DOI: 10.1212/WNL.0000000000011745
Association of Memory Impairment With Concomitant Tau Pathology in Patients With Cerebral Amyloid Angiopathy
Abstract
Objective: Relying on tau-PET imaging, this cross-sectional study explored whether memory impairment is linked to the presence of concomitant tau pathology in individuals with cerebral amyloid angiopathy (CAA).
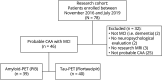
Methods: Forty-six patients with probable CAA underwent a neuropsychological examination and an MRI for quantification of structural markers of cerebral small vessel disease. A subset of these participants also completed a [11C]-Pittsburgh compound B (n = 39) and [18F]-flortaucipir (n = 40) PET for in vivo estimation of amyloid and tau burden, respectively. Participants were classified as amnestic or nonamnestic on the basis of neuropsychological performance. Statistical analyses were performed to examine differences in cognition, structural markers of cerebral small vessel disease, and amyloid- and tau-PET retention between participants with amnestic and those with nonamnestic CAA.
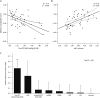
Results: Patients with probable CAA with an amnestic presentation displayed a globally more severe profile of cognitive impairment, smaller hippocampal volume (p < 0.001), and increased tau-PET binding in regions susceptible to Alzheimer disease neurodegeneration (p = 0.003) compared to their nonamnestic counterparts. Amnestic and nonamnestic patients with CAA did not differ on any other MRI markers or on amyloid-PET binding. In a generalized linear model including all evaluated neuroimaging markers, tau-PET retention (β = -0.85, p = 0.001) and hippocampal volume (β = 0.64 p = 0.01) were the only significant predictors of memory performance. The cognitive profile of patients with CAA with an elevated tau-PET retention was distinctly characterized by a significantly lower performance on the memory domain (p = 0.004).
Conclusions: These results suggest that the presence of objective memory impairment in patients with probable CAA could serve as a marker for underlying tau pathology.
Classification of evidence: This study provides Class II evidence that tau-PET retention is related to the presence of objective memory impairment in patients with CAA.
© 2021 American Academy of Neurology.
Figures


References
-
- Thal DR, Griffin WST, de Vos RA, Ghebremedhin E. Cerebral amyloid angiopathy and its relationship to Alzheimer's disease. Acta Neuropathologica 2008;115:599–609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
