Stress hormone-mediated antipredator morphology improves escape performance in amphibian tadpoles
- PMID: 33627747
- PMCID: PMC7904905
- DOI: 10.1038/s41598-021-84052-9
Stress hormone-mediated antipredator morphology improves escape performance in amphibian tadpoles
Abstract
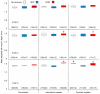
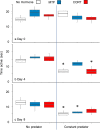
Complete functional descriptions of the induction sequences of phenotypically plastic traits (perception to physiological regulation to response to outcome) should help us to clarify how plastic responses develop and operate. Ranid tadpoles express several plastic antipredator traits mediated by the stress hormone corticosterone, but how they influence outcomes remains uncertain. We investigated how predator-induced changes in the tail morphology of wood frog (Rana sylvatica) tadpoles influenced their escape performance over a sequence of time points when attacked by larval dragonflies (Anax junius). Tadpoles were raised with no predator exposure, chemical cues of dragonflies added once per day, or constant exposure to caged dragonflies crossed with no exogenous hormone added (vehicle control only), exogenous corticosterone, or metyrapone (a corticosteroid synthesis inhibitor). During predation trials, we detected no differences after four days, but after eight days, tadpoles exposed to larval dragonflies and exogenous corticosterone had developed deeper tail muscles and exhibited improved escape performance compared to controls. Treatment with metyrapone blocked the development of a deeper tail muscle and resulted in no difference in escape success. Our findings further link the predator-induced physiological stress response of ranid tadpoles to the development of an antipredator tail morphology that confers performance benefits.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Importance of predator diet cues in responses of larval wood frogs to fish and invertebrate predators.J Chem Ecol. 2001 Jan;27(1):45-51. doi: 10.1023/a:1005663815856. J Chem Ecol. 2001. PMID: 11382066
-
Stress hormones mediate predator-induced phenotypic plasticity in amphibian tadpoles.Proc Biol Sci. 2013 Mar 6;280(1758):20123075. doi: 10.1098/rspb.2012.3075. Print 2013 May 7. Proc Biol Sci. 2013. PMID: 23466985 Free PMC article.
-
Additive effects of freshwater salinization on the predator-induced traits of larval amphibians.Environ Pollut. 2025 May 15;373:126102. doi: 10.1016/j.envpol.2025.126102. Epub 2025 Mar 21. Environ Pollut. 2025. PMID: 40122325
-
Effects of behavioral and morphological plasticity on risk of predation in a Neotropical tadpole.Oecologia. 2004 Sep;141(1):130-8. doi: 10.1007/s00442-004-1652-x. Epub 2004 Jul 20. Oecologia. 2004. PMID: 15278431
-
New effects of Roundup on amphibians: predators reduce herbicide mortality; herbicides induce antipredator morphology.Ecol Appl. 2012 Mar;22(2):634-47. doi: 10.1890/11-0189.1. Ecol Appl. 2012. PMID: 22611860
Cited by
-
Stress hormones mediate developmental plasticity in vertebrates with complex life cycles.Neurobiol Stress. 2021 Feb 2;14:100301. doi: 10.1016/j.ynstr.2021.100301. eCollection 2021 May. Neurobiol Stress. 2021. PMID: 33614863 Free PMC article.
-
Integrating morphology and physiology of the key endocrine organ during tadpole development: The interrenal gland.J Anat. 2022 Dec;241(6):1357-1370. doi: 10.1111/joa.13759. Epub 2022 Sep 2. J Anat. 2022. PMID: 36056596 Free PMC article.
-
Something in the water: aquatic microbial communities influence the larval amphibian gut microbiota, neurodevelopment and behaviour.Proc Biol Sci. 2024 Feb 28;291(2017):20232850. doi: 10.1098/rspb.2023.2850. Epub 2024 Feb 28. Proc Biol Sci. 2024. PMID: 38412968 Free PMC article.
References
-
- Tollrian R, Harvell CD. The Ecology and Evolution of Inducible Defenses. Princeton: Princeton University Press; 1998.
-
- Ohgushi T, Schmitz OJ, Holt RD. Trait-Mediated Indirect Interactions: Ecological and Evolutionary Perspectives. Cambridge: Cambridge University Press; 2013.
-
- Ellers J, Stuefer JF. Frontiers in phenotypic plasticity research: new questions about mechanisms, induced responses, and ecological impacts. Evol. Ecol. 2010;24:523–526. doi: 10.1007/s10682-010-9375-4. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

