H2A.Z overexpression suppresses senescence and chemosensitivity in pancreatic ductal adenocarcinoma
- PMID: 33627784
- PMCID: PMC7979544
- DOI: 10.1038/s41388-021-01664-1
H2A.Z overexpression suppresses senescence and chemosensitivity in pancreatic ductal adenocarcinoma
Abstract
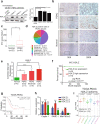
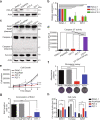
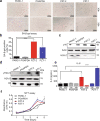
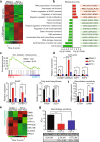
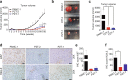
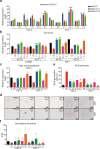
Pancreatic ductal adenocarcinoma (PDAC) is one of the most intractable and devastating malignant tumors. Epigenetic modifications such as DNA methylation and histone modification regulate tumor initiation and progression. However, the contribution of histone variants in PDAC is unknown. Here, we demonstrated that the histone variant H2A.Z is highly expressed in PDAC cell lines and PDAC patients and that its overexpression correlates with poor prognosis. Moreover, all three H2A.Z isoforms (H2A.Z.1, H2A.Z.2.1, and H2A.Z.2.2) are highly expressed in PDAC cell lines and PDAC patients. Knockdown of these H2A.Z isoforms in PDAC cell lines induces a senescent phenotype, cell cycle arrest in phase G2/M, increased expression of cyclin-dependent kinase inhibitor CDKN2A/p16, SA-β-galactosidase activity and interleukin 8 production. Transcriptome analysis of H2A.Z-depleted PDAC cells showed altered gene expression in fatty acid biosynthesis pathways and those that regulate cell cycle and DNA damage repair. Importantly, depletion of H2A.Z isoforms reduces the tumor size in a mouse xenograft model in vivo and sensitizes PDAC cells to gemcitabine. Overexpression of H2A.Z.1 and H2A.Z.2.1 more than H2A.Z.2.2 partially restores the oncogenic phenotype. Therefore, our data suggest that overexpression of H2A.Z isoforms enables cells to overcome the oncoprotective barrier associated with senescence, favoring PDAC tumor grow and chemoresistance. These results make H2A.Z a potential candidate as a diagnostic biomarker and therapeutic target for PDAC.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

