The quiescent endothelium: signalling pathways regulating organ-specific endothelial normalcy
- PMID: 33627876
- PMCID: PMC7903932
- DOI: 10.1038/s41569-021-00517-4
The quiescent endothelium: signalling pathways regulating organ-specific endothelial normalcy
Abstract
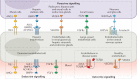
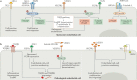
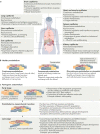
Endothelial cells are at the interface between circulating blood and tissues. This position confers on them a crucial role in controlling oxygen and nutrient exchange and cellular trafficking between blood and the perfused organs. The endothelium adopts a structure that is specific to the needs and function of each tissue and organ and is subject to tissue-specific signalling input. In adults, endothelial cells are quiescent, meaning that they are not proliferating. Quiescence was considered to be a state in which endothelial cells are not stimulated but are instead slumbering and awaiting activating signals. However, new evidence shows that quiescent endothelium is fully awake, that it constantly receives and initiates functionally important signalling inputs and that this state is actively regulated. Signalling pathways involved in the maintenance of functionally quiescent endothelia are starting to be identified and are a combination of endocrine, autocrine, paracrine and mechanical inputs. The paracrine pathways confer a microenvironment on the endothelial cells that is specific to the perfused organs and tissues. In this Review, we present the current knowledge of organ-specific signalling pathways involved in the maintenance of endothelial quiescence and the pathologies associated with their disruption. Linking organ-specific pathways and human vascular pathologies will pave the way towards the development of innovative preventive strategies and the identification of new therapeutic targets.
© 2021. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

