Preclinical Neuropathic Pain Assessment; the Importance of Translatability and Bidirectional Research
- PMID: 33628181
- PMCID: PMC7897667
- DOI: 10.3389/fphar.2020.614990
Preclinical Neuropathic Pain Assessment; the Importance of Translatability and Bidirectional Research
Abstract
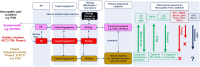
For patients suffering with chronic neuropathic pain the need for suitable novel therapies is imperative. Over recent years a contributing factor for the lack of development of new analgesics for neuropathic pain has been the mismatch of primary neuropathic pain assessment endpoints in preclinical vs. clinical trials. Despite continuous forward translation failures across diverse mechanisms, reflexive quantitative sensory testing remains the primary assessment endpoint for neuropathic pain and analgesia in animals. Restricting preclinical evaluation of pain and analgesia to exclusively reflexive outcomes is over simplified and can be argued not clinically relevant due to the continued lack of forward translation and failures in the clinic. The key to developing new analgesic treatments for neuropathic pain therefore lies in the development of clinically relevant endpoints that can translate preclinical animal results to human clinical trials. In this review we discuss this mismatch of primary neuropathic pain assessment endpoints, together with clinical and preclinical evidence that supports how bidirectional research is helping to validate new clinically relevant neuropathic pain assessment endpoints. Ethological behavioral endpoints such as burrowing and facial grimacing and objective measures such as electroencephalography provide improved translatability potential together with currently used quantitative sensory testing endpoints. By tailoring objective and subjective measures of neuropathic pain the translatability of new medicines for patients suffering with neuropathic pain will hopefully be improved.
Keywords: burrowing; clinical; electroencephalography; endpoints; neuropathic pain; preclinical; quantitative sensory testing; translatability.
Copyright © 2021 Fisher, Lanigan, Upton and Lione.
Conflict of interest statement
NU, ML and AF were employed by the company Transpharmation Ltd. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
Figures


Similar articles
-
A back translation of pregabalin and carbamazepine against evoked and non-evoked endpoints in the rat spared nerve injury model of neuropathic pain.Neuropharmacology. 2013 Oct;73:204-15. doi: 10.1016/j.neuropharm.2013.05.023. Epub 2013 Jun 5. Neuropharmacology. 2013. PMID: 23747575
-
Improving neuropathic pain treatment - by rigorous stratification from bench to bedside.J Neurochem. 2024 Nov;168(11):3699-3714. doi: 10.1111/jnc.15798. Epub 2023 Apr 7. J Neurochem. 2024. PMID: 36852505 Review.
-
Bridging the translational gap: adenosine as a modulator of neuropathic pain in preclinical models and humans.Scand J Pain. 2023 Dec 11;24(1). doi: 10.1515/sjpain-2023-0048. eCollection 2024 Jan 1. Scand J Pain. 2023. PMID: 38070164 Review.
-
Challenges in translational drug research in neuropathic and inflammatory pain: the prerequisites for a new paradigm.Eur J Clin Pharmacol. 2017 Oct;73(10):1219-1236. doi: 10.1007/s00228-017-2301-8. Epub 2017 Sep 11. Eur J Clin Pharmacol. 2017. PMID: 28894907 Free PMC article. Review.
-
Emerging drugs for diabetic peripheral neuropathy and neuropathic pain.Expert Opin Emerg Drugs. 2016 Dec;21(4):393-407. doi: 10.1080/14728214.2016.1257605. Epub 2016 Nov 18. Expert Opin Emerg Drugs. 2016. PMID: 27813425 Review.
Cited by
-
Development of a PET radioligand for α2δ-1 subunit of calcium channels for imaging neuropathic pain.Eur J Med Chem. 2022 Nov 15;242:114688. doi: 10.1016/j.ejmech.2022.114688. Epub 2022 Aug 18. Eur J Med Chem. 2022. PMID: 36031695 Free PMC article.
-
Navigating Preclinical Models and Medications for Peripheral Neuropathy: A Review.Pharmaceuticals (Basel). 2024 Jul 31;17(8):1010. doi: 10.3390/ph17081010. Pharmaceuticals (Basel). 2024. PMID: 39204115 Free PMC article. Review.
-
Effects of 1-methyl-1, 2, 3, 4-tetrahydroisoquinoline on a diabetic neuropathic pain model.Front Pharmacol. 2023 Mar 22;14:1128496. doi: 10.3389/fphar.2023.1128496. eCollection 2023. Front Pharmacol. 2023. PMID: 37033637 Free PMC article.
-
A Review of the Clinical and Therapeutic Implications of Neuropathic Pain.Biomedicines. 2021 Sep 16;9(9):1239. doi: 10.3390/biomedicines9091239. Biomedicines. 2021. PMID: 34572423 Free PMC article. Review.
-
Sensitization of supra-threshold pain responses-Translational aspects and mechanisms.Front Netw Physiol. 2022 Dec 16;2:1078890. doi: 10.3389/fnetp.2022.1078890. eCollection 2022. Front Netw Physiol. 2022. PMID: 36926107 Free PMC article. Review.
References
-
- Agarwal N., Helmstädter J., Rojas D. R., Bali K. K., Gangadharan V., Kuner R. (2018). Evoked hypoalgesia is accompanied by tonic pain and immune cell infiltration in the dorsal root ganglia at late stages of diabetic neuropathy in mice. Mol. Pain 14, 1744806918817975 10.1177/1744806918817975 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

