A Randomized, Double-Blind, Multicenter Clinical Study Comparing the Efficacy and Safety of a Drug Combination of Lopinavir/Ritonavir-Azithromycin, Lopinavir/Ritonavir-Doxycycline, and Azithromycin-Hydroxychloroquine for Patients Diagnosed with Mild to Moderate COVID-19 Infections
- PMID: 33628506
- PMCID: PMC7881739
- DOI: 10.1155/2021/6685921
A Randomized, Double-Blind, Multicenter Clinical Study Comparing the Efficacy and Safety of a Drug Combination of Lopinavir/Ritonavir-Azithromycin, Lopinavir/Ritonavir-Doxycycline, and Azithromycin-Hydroxychloroquine for Patients Diagnosed with Mild to Moderate COVID-19 Infections
Abstract
Background: At the present time, COVID-19 vaccines are at the testing stage, and an effective treatment for COVID-19 incorporating appropriate safety measures remains the most significant obstacle to be overcome. A strategic countermeasure is, therefore, urgently required.
Aim: This study aims to evaluate the efficacy and safety of a combination of lopinavir/ritonavir-azithromycin, lopinavir/ritonavir-doxycycline, and azithromycin-hydroxychloroquine used to treat patients with mild to moderate COVID-19 infections. Setting and Design. This study was conducted at four different clinical study sites in Indonesia. The subjects gave informed consent for their participation and were confirmed as being COVID-19-positive by means of an RT-PCR test. The present study constituted a randomized, double-blind, and multicenter clinical study of patients diagnosed with mild to moderate COVID-19 infection.
Materials and methods: Six treatment groups participated in this study: a Control group administered with a 500 mg dose of azithromycin; Group A which received a 200/50 mg dose of lopinavir/ritonavir and 500 mg of azithromycin; Group B treated with a 200/50 mg dose of lopinavir/ritonavir and 200 mg of doxycycline; Group C administered with 200 mg of hydroxychloroquine and 500 mg of azithromycin; Group D which received a 400/100 mg dose of lopinavir/ritonavir and 500 mg of azithromycin; and Group E treated with a 400/100 mg dose of lopinavir/ritonavir and 200 mg of doxycycline.
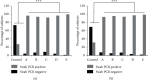
Results: 754 subjects participated in this study: 694 patients (92.4%) who presented mild symptoms and 57 patients (7.6%) classified as suffering from a moderate case of COVID-19. On the third day after treatment, 91.7%-99.2% of the subjects in Groups A-E were confirmed negative by a PCR swab test compared to 26.9% in the Control group. Observation of all groups which experienced a significant decrease in virus load between day 1 and day 7 was undertaken. Other markers, such as CRP and IL-6, were significantly lower in all treatment groups (p < 0.05 and p < 0.0001) than in the Control group. Furthermore, IL-10 and TNF-α levels were significantly elevated in all treatment groups (p < 0.0001). The administration of azithromycin to the Control group increased CRP and IL-6 levels, while reduced IL-10 and TNF-α on day 7 (p < 0.0001) compared with day 1. Decreases in ALT and AST levels were observed in all groups (p < 0.0001). There was an increase in creatinine in the serum level of the Control, C, D, and E groups (p < 0.05), whereas the BUN level was elevated in all groups (p < 0.0001).
Conclusions: The study findings suggest that the administration of lopinavir/ritonavir-doxycycline, lopinavir/ritonavir-azithromycin, and azithromycin-hydroxychloroquine as a dual drug combination produced a significantly rapid PCR conversion rate to negative in three-day treatment of mild to moderate COVID-19 cases. Further studies should involve observation of older patients with severe clinical symptoms in order to collate significant amounts of demographic data.
Copyright © 2021 Purwati et al.
Conflict of interest statement
The authors declare no conflicts of interest regarding this work.
Figures

References
-
- World Health Organisation. WHO Coronavirus Disease (COVID-19) Dashboard. Geneva, Switzerland: World Health Organisation; 2020.
-
- Bruce A., Liang W. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) Geneva, Switzerland: World Health Organisation; 2020. https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mis....
-
- Cascella M., Rajnik M., Cuomo A., Dulebohn S. C., Di Napoli R. Features, Evaluation and Treatment of Coronavirus. San Francisco, CA, USA: StatPearls Publishing; 2020. - PubMed
-
- Centers for Disease Control and Prevention. Clinical Care Guidance. Atlanta, GA, USA: Centers for Disease Control and Prevention; 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-manageme....
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

