The IL-33/ST2 axis affects tumor growth by regulating mitophagy in macrophages and reprogramming their polarization
- PMID: 33628592
- PMCID: PMC7877183
- DOI: 10.20892/j.issn.2095-3941.2020.0211
The IL-33/ST2 axis affects tumor growth by regulating mitophagy in macrophages and reprogramming their polarization
Abstract
Objective: Macrophages are a major component of the tumor microenvironment. M1 macrophages secrete pro-inflammatory factors that inhibit tumor growth and development, whereas tumor-associated macrophages (TAMs) mainly exhibit an M2 phenotype. Our previous studies have shown that the interleukin-33/ST2 (IL-33/ST2) axis is essential for activation of the M1 phenotype. This study investigates the role of the IL-33/ST2 axis in TAMs, its effects on tumor growth, and whether it participates in the mutual conversion between the M1 and M2 phenotypes.
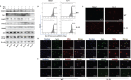
Methods: Bone marrow-derived macrophages were extracted from wildtype, ST2 knockout (ST2-/-), and Il33-overexpressing mice and differentiated with IL-4. The mitochondrial and lysosomal number and location, and the expression of related proteins were used to analyze mitophagy. Oxygen consumption rates and glucose and lactate levels were measured to reveal metabolic changes.
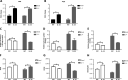
Results: The IL-33/ST2 axis was demonstrated to play an important role in the metabolic conversion of macrophages from OXPHOS to glycolysis by altering mitophagy levels. The IL-33/ST2 axis promoted enhanced cell oxidative phosphorylation, thereby further increasing M2 polarization gene expression and ultimately promoting tumor growth (P < 0.05) (Figure 4). This metabolic shift was not due to mitochondrial damage, because the mitochondrial membrane potential was not significantly altered by IL-4 stimulation or ST2 knockout; however, it might be associated with the mTOR activity.
Conclusions: These results clarify the interaction between the IL-33/ST2 pathway and macrophage polarization, and may pave the way to the development of new cancer immunotherapies targeting the IL-33/ST2 axis.
Keywords: IL-33/ST2; glucose metabolism; macrophage polarization; mitophagy; tumor microenvironment.
Copyright: © 2021, Cancer Biology & Medicine.
Conflict of interest statement
Conflicts of interest statement No potential conflicts of interest are disclosed.
Figures





References
-
- Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, et al. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18:349–55. - PubMed
-
- Locati M, Mantovani A, Sica A. Macrophage activation and polarization as an adaptive component of innate immunity. Adv Immunol. 2013;120:163–84. - PubMed
-
- Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–39. - PubMed
-
- Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–61. - PubMed
-
- Yue ZQ, Liu YP, Ruan JS, Zhou L, Lu Y. Tumor-associated macrophages: a novel potential target for cancer treatment. Chin Med J (Engl) 2012;125:3305–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
