Diagnostic value of 5 serum biomarkers for hepatocellular carcinoma with different epidemiological backgrounds: A large-scale, retrospective study
- PMID: 33628599
- PMCID: PMC7877174
- DOI: 10.20892/j.issn.2095-3941.2020.0207
Diagnostic value of 5 serum biomarkers for hepatocellular carcinoma with different epidemiological backgrounds: A large-scale, retrospective study
Abstract
Objective: Hepatocellular carcinoma (HCC) is a lethal global disease that requires an accurate diagnosis. We assessed the potential of 5 serum biomarkers (AFP, AFU, GGT-II, GPC3, and HGF) in the diagnosis of HCC.
Methods: In this retrospective study, we measured the serum levels of each biomarker using ELISAs in 921 participants, including 298 patients with HCC, 154 patients with chronic hepatitis (CH), 122 patients with liver cirrhosis (LC), and 347 healthy controls from 3 hospitals. Patients negative for hepatitis B surface antigen and hepatitis C antibody (called "NBNC-HCC") and patients positive for the above indices (called "HBV-HCC and HCV-HCC") were enrolled. The selected diagnostic model was constructed using a training cohort (n = 468), and a validation cohort (n = 453) was used to validate our results. Receiver operating characteristic analysis was used to evaluate the diagnostic accuracy.
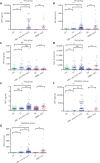
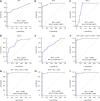
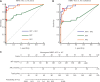
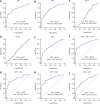
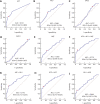
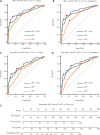
Results: The α-L-fucosidase (AFU)/α-fetoprotein (AFP) combination was best able to distinguish NBNC-HCC [area under the curve: 0.986 (95% confidence interval: 0.958-0.997), sensitivity: 92.6%, specificity: 98.9%] from healthy controls in the test cohort. For screening populations at risk of developing HCC (CH and LC), the AFP/AFU combination improved the diagnostic specificity for early-stage HCC [area under the curve: 0.776 (0.712-0.831), sensitivity: 52.5%, specificity: 91.6% in the test group]. In all-stage HBV-HCC and HCV-HCC, AFU was also the best candidate biomarker combined with AFP [area under the curve: 0.835 (0.784-0.877), sensitivity 69.1%, specificity: 87.4% in the test group]. All results were verified in the validation group.
Conclusions: The AFP/AFU combination could be used to identify NBNC-HCC from healthy controls and hepatitis-related HCC from at-risk patients.
Keywords: AFP; AFU; Hepatocellular carcinoma; biomarker; serum.
Copyright: © 2021, Cancer Biology & Medicine.
Conflict of interest statement
Conflict of interest statement No potential conflicts of interest are disclosed.
Figures
Similar articles
-
Evaluation of squamous cell carcinoma antigen-immunoglobulin M complex (SCCA-IGM) and alpha-L-fucosidase (AFU) as novel diagnostic biomarkers for hepatocellular carcinoma.Tumour Biol. 2014 Nov;35(11):11559-64. doi: 10.1007/s13277-014-2467-y. Epub 2014 Aug 17. Tumour Biol. 2014. PMID: 25129443
-
A Novel Online Calculator Based on Serum Biomarkers to Detect Hepatocellular Carcinoma among Patients with Hepatitis B.Clin Chem. 2019 Dec;65(12):1543-1553. doi: 10.1373/clinchem.2019.308965. Epub 2019 Oct 31. Clin Chem. 2019. PMID: 31672853 Clinical Trial.
-
Diagnostic Value of Combined Serum Marker Tests in Hepatitis B Virus-Associated Hepatocellular Carcinoma.Altern Ther Health Med. 2024 May;30(5):168-173. Altern Ther Health Med. 2024. PMID: 37917888
-
Abdominal ultrasound and alpha-foetoprotein for the diagnosis of hepatocellular carcinoma in adults with chronic liver disease.Cochrane Database Syst Rev. 2021 Apr 15;4(4):CD013346. doi: 10.1002/14651858.CD013346.pub2. Cochrane Database Syst Rev. 2021. PMID: 33855699 Free PMC article.
-
Diagnostic value of alpha-L-fucosidase for hepatocellular carcinoma: a meta-analysis.Tumour Biol. 2014 May;35(5):3953-60. doi: 10.1007/s13277-013-1563-8. Epub 2014 Jan 7. Tumour Biol. 2014. PMID: 24395655
Cited by
-
Unique Features of Hepatitis B Virus-Related Hepatocellular Carcinoma in Pathogenesis and Clinical Significance.Cancers (Basel). 2021 May 18;13(10):2454. doi: 10.3390/cancers13102454. Cancers (Basel). 2021. PMID: 34070067 Free PMC article. Review.
-
A meta-analysis and of clinical values of 11 blood biomarkers, such as AFP, DCP, and GP73 for diagnosis of hepatocellular carcinoma.Ann Med. 2023 Dec;55(1):42-61. doi: 10.1080/07853890.2022.2153163. Ann Med. 2023. Retraction in: Ann Med. 2024 Dec;56(1):2415160. doi: 10.1080/07853890.2024.2415160. PMID: 36476015 Free PMC article. Retracted.
-
Efficacy and safety of sequential therapy with sorafenib and regorafenib for advanced hepatocellular carcinoma: a two-center study in China.J Gastrointest Oncol. 2022 Jun;13(3):1266-1277. doi: 10.21037/jgo-22-397. J Gastrointest Oncol. 2022. PMID: 35837206 Free PMC article.
-
Identification of prognostic factors and nomogram model for patients with advanced lung cancer receiving immune checkpoint inhibitors.PeerJ. 2022 Dec 15;10:e14566. doi: 10.7717/peerj.14566. eCollection 2022. PeerJ. 2022. PMID: 36540802 Free PMC article.
-
Current status and new directions for hepatocellular carcinoma diagnosis.Liver Res. 2024 Dec 5;8(4):218-236. doi: 10.1016/j.livres.2024.12.001. eCollection 2024 Dec. Liver Res. 2024. PMID: 39958920 Free PMC article. Review.
References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. - PubMed
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
