A Widely Applicable Dual Catalytic System for Cross-Electrophile Coupling Enabled by Mechanistic Studies
- PMID: 33628617
- PMCID: PMC7899151
- DOI: 10.1021/acscatal.0c03237
A Widely Applicable Dual Catalytic System for Cross-Electrophile Coupling Enabled by Mechanistic Studies
Abstract
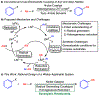
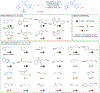
A dual catalytic system for cross-electrophile coupling reactions between aryl halides and alkyl halides that features a Ni catalyst, a Co cocatalyst, and a mild homogeneous reductant is described. Mechanistic studies indicate that the Ni catalyst activates the aryl halide, while the Co cocatalyst activates the alkyl halide. This allows the system to be rationally optimized for a variety of substrate classes by simply modifying the loadings of the Ni and Co catalysts based on the reaction product profile. For example, the coupling of aryl bromides and aryl iodides with alkyl bromides, alkyl iodides, and benzyl chlorides is demonstrated using the same Ni and Co catalysts under similar reaction conditions but with different optimal catalyst loadings in each case. Our system is tolerant of numerous functional groups and is capable of coupling heteroaryl halides, di-ortho-substituted aryl halides, pharmaceutically relevant druglike aryl halides, and a diverse range of alkyl halides. Additionally, the dual catalytic platform facilitates a series of selective one-pot three-component cross-electrophile coupling reactions of bromo(iodo)arenes with two distinct alkyl halides. This demonstrates the unique level of control that the platform provides and enables the rapid generation of molecular complexity. The system can be readily utilized for a wide range of applications as all reaction components are commercially available, the reaction is scalable, and toxic amide-based solvents are not required. It is anticipated that this strategy, as well as the underlying mechanistic framework, will be generalizable to other cross-electrophile coupling reactions.
Keywords: cross-electrophile coupling; mechanism; medicinal chemistry; nickel; synthetic methods.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
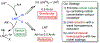
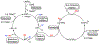


Similar articles
-
Methods and Mechanisms for Cross-Electrophile Coupling of Csp(2) Halides with Alkyl Electrophiles.Acc Chem Res. 2015 Jun 16;48(6):1767-75. doi: 10.1021/acs.accounts.5b00057. Epub 2015 May 26. Acc Chem Res. 2015. PMID: 26011466 Free PMC article.
-
Nickel-Catalyzed Electrochemical Cross-Electrophile C(sp2)-C(sp3) Coupling via a NiII Aryl Amido Intermediate.Angew Chem Int Ed Engl. 2024 Sep 16;63(38):e202407118. doi: 10.1002/anie.202407118. Epub 2024 Aug 13. Angew Chem Int Ed Engl. 2024. PMID: 38849318
-
Nickel-Catalyzed Cross-Electrophile Coupling with Organic Reductants in Non-Amide Solvents.Chemistry. 2016 Aug 8;22(33):11564-7. doi: 10.1002/chem.201602668. Epub 2016 Jul 8. Chemistry. 2016. PMID: 27273457
-
Electroreductive Cross-Electrophile Coupling (eXEC) Reactions.Angew Chem Int Ed Engl. 2023 Nov 6;62(45):e202306679. doi: 10.1002/anie.202306679. Epub 2023 Jul 12. Angew Chem Int Ed Engl. 2023. PMID: 37327185 Review.
-
Direct vs Indirect Grafting of Alkyl and Aryl Halides.Chemphyschem. 2021 Sep 15;22(18):1844-1849. doi: 10.1002/cphc.202100296. Epub 2021 Jul 29. Chemphyschem. 2021. PMID: 34125990 Review.
Cited by
-
Zinc-Free, Scalable Reductive Cross-Electrophile Coupling Driven by Electrochemistry in an Undivided Cell.ACS Catal. 2022 Oct 21;12(20):12617-12626. doi: 10.1021/acscatal.2c03033. Epub 2022 Oct 3. ACS Catal. 2022. PMID: 37065181 Free PMC article.
-
In-Situ Bromination Enables Formal Cross-Electrophile Coupling of Alcohols with Aryl and Alkenyl Halides.ACS Catal. 2022 Jan 7;12(1):580-586. doi: 10.1021/acscatal.1c05208. Epub 2021 Dec 21. ACS Catal. 2022. PMID: 35386235 Free PMC article.
-
Tunable and Practical Homogeneous Organic Reductants for Cross-Electrophile Coupling.J Am Chem Soc. 2021 Dec 15;143(49):21024-21036. doi: 10.1021/jacs.1c10932. Epub 2021 Nov 30. J Am Chem Soc. 2021. PMID: 34846142 Free PMC article.
-
Cross-Electrophile Coupling: Principles, Methods, and Applications in Synthesis.Chem Rev. 2024 Dec 11;124(23):13397-13569. doi: 10.1021/acs.chemrev.4c00524. Epub 2024 Nov 26. Chem Rev. 2024. PMID: 39591522 Free PMC article. Review.
-
High-Throughput Photo- and Electrochemical sp2-sp3 Cross-Electrophile Coupling to Access Novel Tedizolid Analogs.ACS Med Chem Lett. 2023 May 1;14(5):666-671. doi: 10.1021/acsmedchemlett.2c00542. eCollection 2023 May 11. ACS Med Chem Lett. 2023. PMID: 37197470 Free PMC article.
References
-
- Everson DA; Weix DJ Cross-Electrophile Coupling: Principles of Reactivity and Selectivity. J. Org. Chem 2014, 79, 4793–4798. - PMC - PubMed
- Knappke CEI; Grupe S; Gärtner D; Corpet M; Gosmini C; Jacobi von Wangelin A Reductive Cross-Coupling Reactions Between Two Electrophiles. Chem. – Eur. J 2014, 20, 6828–6842. - PubMed
- Moragas T; Correa A; Martin R Metal-Catalyzed Reductive Coupling Reactions of Organic Halides with Carbonyl-Type Compounds. Chem. – Eur. J 2014, 20, 8242–8258. - PubMed
- Gu J; Wang X; Xue W; Gong H Nickel-Catalyzed Reductive Coupling of Alkyl Halides with Other Electrophiles: Concept and Mechanistic Considerations. Org. Chem. Front 2015, 2, 1411–1421.
- Weix DJ Methods and Mechanisms for Cross-Electrophile Coupling of Csp2 Halides with Alkyl Electrophiles. Acc. Chem. Res 2015, 48, 1767–1775. - PMC - PubMed
- Richmond E; Moran J Recent Advances in Nickel Catalysis Enabled by Stoichiometric Metallic Reducing Agents. Synthesis 2018, 50, 499–513.
- Tortajada A; Juliá-Hernández F; Börjesson M; Moragas T; Martin R Transition-Metal-Catalyzed Carboxylation Reactions with Carbon Dioxide. Angew. Chem. Int. Ed 2018, 57, 15948–15982. - PubMed
- Campeau L-C; Hazari N Cross-Coupling and Related Reactions: Connecting Past Success to the Development of New Reactions for the Future. Organometallics 2019, 38, 3–35. - PMC - PubMed
- Poremba KE; Dibrell SE; Reisman SE Nickel-Catalyzed Enantioselective Reductive Cross-Coupling Reactions. ACS Catal. 2020, 8237–8246. - PMC - PubMed
-
- For leading references, see.
- Everson DA; Shrestha R; Weix DJ Nickel-Catalyzed Reductive Cross-Coupling of Aryl Halides with Alkyl Halides. J. Am. Chem. Soc 2010, 132, 920–921. - PubMed
- Everson DA; Jones BA; Weix DJ Replacing Conventional Carbon Nucleophiles with Electrophiles: Nickel-Catalyzed Reductive Alkylation of Aryl Bromides and Chlorides. J. Am. Chem. Soc 2012, 134, 6146–6159. - PMC - PubMed
- Wotal AC; Weix DJ Synthesis of Functionalized Dialkyl Ketones from Carboxylic Acid Derivatives and Alkyl Halides. Org. Lett 2012, 14, 1476–1479. - PMC - PubMed
- Cherney AH; Kadunce NT; Reisman SE Catalytic Asymmetric Reductive Acyl Cross-Coupling: Synthesis of Enantioenriched Acyclic α,α-Disubstituted Ketones. J. Am. Chem. Soc 2013, 135, 7442–7445. - PubMed
- Cherney AH; Reisman SE Nickel-Catalyzed Asymmetric Reductive Cross-Coupling Between Vinyl and Benzyl Electrophiles. J. Am. Chem. Soc 2014, 136, 14365–14368. - PMC - PubMed
- Molander GA; Traister KM; O’Neill BT Reductive Cross-Coupling of Nonaromatic, Heterocyclic Bromides with Aryl and Heteroaryl Bromides. J. Org. Chem 2014, 79, 5771–5780. - PubMed
- Molander GA; Wisniewski SR; Traister KM Reductive Cross-Coupling of 3-Bromo-2,1-borazaronaphthalenes with Alkyl Iodides. Org. Lett 2014, 16, 3692–3695. - PMC - PubMed
- Zhao C; Jia X; Wang X; Gong H Ni-Catalyzed Reductive Coupling of Alkyl Acids with Unactivated Tertiary Alkyl and Glycosyl Halides. J. Am. Chem. Soc 2014, 136, 17645–17651. - PubMed
- Zhao Y; Weix DJ Nickel-Catalyzed Regiodivergent Opening of Epoxides with Aryl Halides: Co-Catalysis Controls Regioselectivity. J. Am. Chem. Soc 2014, 136, 48–51. - PMC - PubMed
- Zhao Y; Weix DJ Enantioselective Cross-Coupling of meso-Epoxides with Aryl Halides. J. Am. Chem. Soc 2015, 137, 3237–3240. - PMC - PubMed
- Arendt KM; Doyle AG Dialkyl Ether Formation by Nickel-Catalyzed Cross-Coupling of Acetals and Aryl Iodides. Angew. Chem. Int. Ed 2015, 54, 9876–9880. - PMC - PubMed
- Kadunce NT; Reisman SE Nickel-Catalyzed Asymmetric Reductive Cross-Coupling between Heteroaryl Iodides and α-Chloronitriles. J. Am. Chem. Soc 2015, 137, 10480–10483. - PMC - PubMed
- Molander GA; Traister KM; O’Neill BT Engaging Nonaromatic, Heterocyclic Tosylates in Reductive Cross-Coupling with Aryl and Heteroaryl Bromides. J. Org. Chem 2015, 80, 2907–2911. - PubMed
- Wang X; Wang S; Xue W; Gong H Nickel-Catalyzed Reductive Coupling of Aryl Bromides with Tertiary Alkyl Halides. J. Am. Chem. Soc 2015, 137, 11562–11565. - PubMed
- Hu L; Liu X; Liao X Nickel-Catalyzed Methylation of Aryl Halides with Deuterated Methyl Iodide. Angew. Chem. Int. Ed 2016, 55, 9743–9747. - PubMed
- Huihui KMM; Caputo JA; Melchor Z; Olivares AM; Spiewak AM; Johnson KA; DiBenedetto TA; Kim S; Ackerman LKG; Weix DJ Decarboxylative Cross-Electrophile Coupling of N-Hydroxyphthalimide Esters with Aryl Iodides. J. Am. Chem. Soc 2016, 138, 5016–5019. - PMC - PubMed
- Johnson KA; Biswas S; Weix DJ Cross-Electrophile Coupling of Vinyl Halides with Alkyl Halides. Chem. – Eur. J 2016, 22, 7399–7402. - PMC - PubMed
- Konev MO; Hanna LE; Jarvo ER Intra- and Intermolecular Nickel-Catalyzed Reductive Cross-Electrophile Coupling Reactions of Benzylic Esters with Aryl Halides. Angew. Chem. Int. Ed 2016, 55, 6730–6733. - PubMed
- Liu J; Ren Q; Zhang X; Gong H Preparation of Vinyl Arenes by Nickel-Catalyzed Reductive Coupling of Aryl Halides with Vinyl Bromides. Angew. Chem. Int. Ed 2016, 55, 15544–15548. - PubMed
- Chen F; Chen K; Zhang Y; He Y; Wang Y-M; Zhu S Remote Migratory Cross-Electrophile Coupling and Olefin Hydroarylation Reactions Enabled by in Situ Generation of NiH. J. Am. Chem. Soc 2017, 139, 13929–13935. - PubMed
- He Y; Cai Y; Zhu S Mild and Regioselective Benzylic C–H Functionalization: Ni-Catalyzed Reductive Arylation of Remote and Proximal Olefins. J. Am. Chem. Soc 2017, 139, 1061–1064. - PubMed
- Poremba KE; Kadunce NT; Suzuki N; Cherney AH; Reisman SE Nickel-Catalyzed Asymmetric Reductive Cross-Coupling To Access 1,1-Diarylalkanes. J. Am. Chem. Soc 2017, 139, 5684–5687. - PMC - PubMed
- Woods BP; Orlandi M; Huang C-Y; Sigman MS; Doyle AG Nickel-Catalyzed Enantioselective Reductive Cross-Coupling of Styrenyl Aziridines. J. Am. Chem. Soc 2017, 139, 5688–5691. - PubMed
- Schneider P; Schneider G Privileged Structures Revisited. Angew. Chem. Int. Ed 2017, 56, 7971–7974. - PMC - PubMed
- Peng L; Li Y; Li Y; Wang W; Pang H; Yin G Ligand-Controlled Nickel-Catalyzed Reductive Relay Cross-Coupling of Alkyl Bromides and Aryl Bromides. ACS Catal 2018, 8, 310–313.
- Wang X; Ma G; Peng Y; Pitsch CE; Moll BJ; Ly TD; Wang X; Gong H Ni-Catalyzed Reductive Coupling of Electron-Rich Aryl Iodides with Tertiary Alkyl Halides. J. Am. Chem. Soc 2018, 140, 14490–14497. - PubMed
- Xu C; Guo W-H; He X; Guo Y-L; Zhang X-Y; Zhang X Difluoromethylation of (hetero)aryl Chlorides with Chlorodifluoromethane Catalyzed by Nickel. Nature Commun 2018, 9, 1170. - PMC - PubMed
- Liao J; Basch CH; Hoerrner ME; Talley MR; Boscoe BP; Tucker JW; Garnsey MR; Watson MP Deaminative Reductive Cross-Electrophile Couplings of Alkylpyridinium Salts and Aryl Bromides. Org. Lett 2019, 21, 2941–2946. - PMC - PubMed
- Ni S; Li C-X; Mao Y; Han J; Wang Y; Yan H; Pan Y Ni-Catalyzed Deaminative Cross-Electrophile Coupling of Katritzky Salts with Halides via C–N Bond Activation. Sci. Adv 2019, 5, 9516. - PMC - PubMed
- Ni S; Padial NM; Kingston C; Vantourout JC; Schmitt DC; Edwards JT; Kruszyk MM; Merchant RR; Mykhailiuk PK; Sanchez BB; Yang S; Perry MA; Gallego GM; Mousseau JJ; Collins MR; Cherney RJ; Lebed PS; Chen JS; Qin T; Baran PS A Radical Approach to Anionic Chemistry: Synthesis of Ketones, Alcohols, and Amines. J. Am. Chem. Soc 2019, 141, 6726–6739. - PMC - PubMed
- Tian Z-X; Qiao J-B; Xu G-L; Pang X; Qi L; Ma W-Y; Zhao Z-Z; Duan J; Du Y-F; Su P; Liu X-Y; Shu X-Z Highly Enantioselective Cross-Electrophile Aryl-Alkenylation of Unactivated Alkenes. J. Am. Chem. Soc 2019, 141, 7637–7643. - PubMed
- Wang J; Cary BP; Beyer PD; Gellman SH; Weix DJ Ketones from Nickel-Catalyzed Decarboxylative, Non-Symmetric Cross-Electrophile Coupling of Carboxylic Acid Esters. Angew. Chem. Int. Ed 2019, 58, 12081–12085. - PMC - PubMed
- Ye Y; Chen H; Sessler JL; Gong H Zn-Mediated Fragmentation of Tertiary Alkyl Oxalates Enabling Formation of Alkylated and Arylated Quaternary Carbon Centers. J. Am. Chem. Soc 2019, 141, 820–824. - PubMed
- Yue H; Zhu C; Shen L; Geng Q; Hock KJ; Yuan T; Cavallo L; Rueping M Nickel-Catalyzed C–N Bond Activation: Activated Primary Amines as Alkylating Reagents in Reductive Cross-Coupling. Chem. Sci 2019, 10, 4430–4435. - PMC - PubMed
- Duan J; Du Y-F; Pang X; Shu X-Z Ni-Catalyzed Cross-Electrophile Coupling Between Vinyl/Aryl and Alkyl Sulfonates: Synthesis of Cycloalkenes and Modification of Peptides. Chem. Sci 2019, 10, 8706–8712. - PMC - PubMed
- Kim S; Goldfogel MJ; Gilbert MM; Weix DJ Nickel-Catalyzed Cross-Electrophile Coupling of Aryl Chlorides with Primary Alkyl Chlorides. J. Am. Chem. Soc 2020, 142, 9902–9907. - PMC - PubMed
- Parasram M; Shields BJ; Ahmad O; Knauber T; Doyle AG Regioselective Cross-Electrophile Coupling of Epoxides and (Hetero)aryl Iodides via Ni/Ti/Photoredox Catalysis. ACS Catal 2020, 10, 5821–5827. - PMC - PubMed
- Wang J; Hoerrner ME; Watson MP; Weix DJ Nickel-Catalyzed Synthesis of Dialkyl Ketones from the Coupling of N-Alkyl Pyridinium Salts with Activated Carboxylic Acids. Angew. Chem. Int. Ed 2020, 59, 13484–13489. - PMC - PubMed
-
- Biswas S; Weix DJ Mechanism and Selectivity in Nickel-Catalyzed Cross-Electrophile Coupling of Aryl Halides with Alkyl Halides. J. Am. Chem. Soc 2013, 135, 16192–16197. - PMC - PubMed
- Diccianni JB; Katigbak J; Hu C; Diao T Mechanistic Characterization of (Xantphos)Ni(I)-Mediated Alkyl Bromide Activation: Oxidative Addition, Electron Transfer, or Halogen-Atom Abstraction. J. Am. Chem. Soc 2019, 141, 1788–1796. - PubMed
- Lin Q; Diao T Mechanism of Ni-Catalyzed Reductive 1,2-Dicarbofunctionalization of Alkenes. J. Am. Chem. Soc 2019, 141, 17937–17948. - PMC - PubMed
- Mohadjer Beromi M; Brudvig GW; Hazari N; Lant HMC; Mercado BQ Synthesis and Reactivity of Paramagnetic Nickel Polypyridyl Complexes Relevant to C(sp2)–C(sp3) Coupling Reactions. Angew. Chem. Int. Ed 2019, 58, 6094–6098. - PMC - PubMed
-
- Isbrandt ES; Sullivan RJ; Newman SG High Throughput Strategies for the Discovery and Optimization of Catalytic Reactions. Angew. Chem. Int. Ed 2019, 58, 7180–7191. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
