Effect of Propolis on moderate persistent asthma: A phase two randomized, double blind, controlled clinical trial
- PMID: 33628717
- PMCID: PMC7885004
Effect of Propolis on moderate persistent asthma: A phase two randomized, double blind, controlled clinical trial
Abstract
Objective: The aims of this study was to determine the effect of Propolis (resinous mixture that honey bees produce by mixing saliva and beeswax) on clinical and physiological findings of moderate persistent asthma.
Materials and methods: Fifty-two subjects aged 44.6±18.5 years old with moderate asthma and Forced expiratory volume in 1 second (FEV1) 60-79% of predicted, were enrolled in this clinical trial. We randomly allocated subjects to receive either propolis (75 mg three times a day) or a matched placebo for one month. Primary outcome was Asthma control test (ACT) score and secondary outcomes included dyspnea, spirometry, fractional exhaled nitric oxide (FENO) and sputum cytology including inflammatory cell. Sputum induction was done by hypertonic saline and cytology slides were stained by Papanicolaou stain.
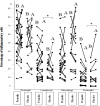
Results: Clinical findings significantly improved after the treatment. ACT scores significantly increased by using propolis (12.8±5.5 before and 18.1±4.99 after the trial), which was significantly higher than the placebo group (14.4±6.6 after the trial). The most significant physiological improvements were significant increases in FEV1, FV1/Forced vital capacity and expiratory flows. FENO showed significant decreases in the propolis group but increases in the placebo group. Cytological examination of sputum showed that the pattern of inflammation was eosinophilic in 44% subjects with an average eosinophil of 7.2±1.01%. Eosinophilia significantly decreased (p<0.05) by using propolis (7.2±1.01 and 4.3±3.1%, before and after treatment, respectively), but it significantly increased (p<0.04) in the placebo group (5.5±2.8, and 11.1±6.6%, before and after treatment, respectively).
Conclusion: Propolis improved the clinical and physiological findings of moderate persistent asthma, and it was able to suppress eosinophilic inflammation.
Keywords: Asthma; Caffeic acid phenethyl ester; Naringenin; Propolis; Quercetin.
Figures
References
-
- Burdock G. Review of the biological properties and toxicity of bee propolis (propolis) Food Chem toxicol. 1998;36:347–363. - PubMed
-
- Djukanović R, Sterk P, Fahy J, Hargreave F. Standardised methodology of sputum induction and processing. Eur Res J. 2002;20:1s–2s. - PubMed
-
- de Farias JH, Reis AS, Araújo MA, Araújo MJ, Assunção AK, de Farias JC, Fialho EMS, Silva LA, Conceição Costa G, Meireles Guerra RN, Sousa Ribeiro MN, Fernandes do Nascimento FR. Effects of stingless bee propolis on experimental asthma. Evid-Base Complement Alternat Med. 2014;2014. doi - PMC - PubMed
-
- Iwamura C, Shinoda K, Yoshimura M, Watanabe Y, Obata A, Nakayama T. Naringenin chalcone suppresses allergic asthma by inhibiting the type-2 function of CD4 T cells. Allergol Int. 2010;59:67–73. - PubMed
-
- Juniper E, O′ byrne P, Guyatt G, Ferrie P, King D. Development and validation of a questionnaire to measure asthma control. Eur res j. 1999;14:902–907. - PubMed
LinkOut - more resources
Full Text Sources