Anti-IgE for the Treatment of Chronic Urticaria
- PMID: 33628747
- PMCID: PMC7898214
- DOI: 10.2147/ITT.S261416
Anti-IgE for the Treatment of Chronic Urticaria
Abstract
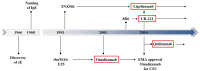
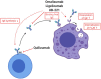
Urticaria and angioedema are very common. Management of chronic urticaria subtypes, which usually persist for many years, is challenging. Recent years have demonstrated that targeting IgE with antibodies provides a safe and efficient treatment approach. Whilst several anti-IgE antibodies have been developed, omalizumab is currently the only one approved for use. International and national guidelines recommend its use after failure of antihistamines at standard and increased dose. Whilst not yet approved, many new anti-IgE approaches are currently being investigated in pre-clinical studies or clinical trials. This non-systematic focused review summarizes current knowledge of omalizumab and other anti-IgE biologics in chronic urticaria using data extracted from PubMed, Google Scholar and clinical trial databases, clinicaltrials.gov and clinicaltrials.eu. For adults, there is good evidence from randomized clinical trials and real-world data that symptomatic treatment with omalizumab is efficacious and safe in chronic spontaneous urticaria (CSU), whereas evidence in chronic inducible urticaria (CINDU) and special populations is limited. Easy-to-use tools to identify non-responders and predict the required duration of treatment have not been established yet. Phase 2 b results of ligelizumab have not only demonstrated efficacy and safety but also superiority to omalizumab. Indeed, there is preliminary evidence that omalizumab non- or partial responders benefit from ligelizumab. Whereas further development of quilizumab was discontinued, other approaches, eg UB-221 or DARPins are under investigation. Anti-IgE treatment with omalizumab represents a landmark in the treatment of chronic urticaria, with and without angioedema, and there is light on the horizon suggesting success may come with various next-generation anti-IgE approaches.
Keywords: UB-221; anti-IgE therapy; ligelizumab; monoclonal antibody; omalizumab; quilizumab; urticaria.
© 2021 Wedi and Traidl.
Conflict of interest statement
During the last 3 years, Bettina Wedi has received honorary for educational lectures/advisory boards from ALK-Abelló, Dr. Pfleger, HAL Allergy Novartis, Shire/Takeda, and was the recipient of a research grant from Shire/Takeda. Bettina Wedi had and has a role as Lead or Principle Investigator in clinical trials of Novartis regarding Omalizumab and Ligelizumab. Stephan Traidl has nothing to declare. The authors report no other conflicts of interest in this work.
Figures

Similar articles
-
Current and Future Approaches in Management of Chronic Spontaneous Urticaria Using Anti-IgE Antibodies.Medicina (Kaunas). 2022 Jun 17;58(6):816. doi: 10.3390/medicina58060816. Medicina (Kaunas). 2022. PMID: 35744079 Free PMC article. Review.
-
Ligelizumab for Chronic Spontaneous Urticaria.N Engl J Med. 2019 Oct 3;381(14):1321-1332. doi: 10.1056/NEJMoa1900408. N Engl J Med. 2019. PMID: 31577874 Clinical Trial.
-
Ligelizumab for the treatment of chronic spontaneous urticaria.Expert Opin Biol Ther. 2020 Aug;20(8):853-861. doi: 10.1080/14712598.2020.1767061. Epub 2020 May 19. Expert Opin Biol Ther. 2020. PMID: 32380864 Review.
-
IgE-neutralizing UB-221 mAb, distinct from omalizumab and ligelizumab, exhibits CD23-mediated IgE downregulation and relieves urticaria symptoms.J Clin Invest. 2022 Aug 1;132(15):e157765. doi: 10.1172/JCI157765. J Clin Invest. 2022. PMID: 35912861 Free PMC article.
-
Omalizumab treatment and outcomes in Chinese patients with chronic spontaneous urticaria, chronic inducible urticaria, or both.World Allergy Organ J. 2021 Jan 5;14(1):100501. doi: 10.1016/j.waojou.2020.100501. eCollection 2021 Jan. World Allergy Organ J. 2021. PMID: 33510832 Free PMC article.
Cited by
-
Identification of key genes and molecular mechanisms of chronic urticaria based on bioinformatics.Skin Res Technol. 2024 Apr;30(4):e13624. doi: 10.1111/srt.13624. Skin Res Technol. 2024. PMID: 38558219 Free PMC article.
-
β-arrestin-1 and β-arrestin-2 Restrain MRGPRX2-Triggered Degranulation and ERK1/2 Activation in Human Skin Mast Cells.Front Allergy. 2022 Jul 15;3:930233. doi: 10.3389/falgy.2022.930233. eCollection 2022. Front Allergy. 2022. PMID: 35910860 Free PMC article.
-
New Mechanistic Advances in FcεRI-Mast Cell-Mediated Allergic Signaling.Clin Rev Allergy Immunol. 2022 Dec;63(3):431-446. doi: 10.1007/s12016-022-08955-9. Epub 2022 Oct 17. Clin Rev Allergy Immunol. 2022. PMID: 36251242 Free PMC article. Review.
-
Prescription Behaviour and Barriers to Prescription of Biologicals for Treatment of Chronic Inflammatory Skin Diseases in Dermatological Practice in Two German Federal States.Acta Derm Venereol. 2021 Sep 28;101(9):adv00560. doi: 10.2340/00015555-3901. Acta Derm Venereol. 2021. PMID: 34427313 Free PMC article.
-
A 10-Year Real-World Analysis of Omalizumab Use in Chronic Spontaneous Urticaria Patients at a Dermatology Clinic.Sisli Etfal Hastan Tip Bul. 2025 Feb 7;59(2):194-199. doi: 10.14744/SEMB.2025.79059. eCollection 2025. Sisli Etfal Hastan Tip Bul. 2025. PMID: 40756290 Free PMC article.
References
-
- Wedi B. Urticaria and angioedema In: Plewig G, French L, Ruzicka T, Kaufmann R, Hertl M, editors. Braun-falco’s dermatologe. Berlin, Heidelberg: Springer Germany; 2020. doi:10.1007/978-3-662-58713-3_29-1. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

