Application of Plasmonic Metal Nanoparticles in TiO2-SiO2 Composite as an Efficient Solar-Activated Photocatalyst: A Review Paper
- PMID: 33628762
- PMCID: PMC7897925
- DOI: 10.3389/fchem.2020.568063
Application of Plasmonic Metal Nanoparticles in TiO2-SiO2 Composite as an Efficient Solar-Activated Photocatalyst: A Review Paper
Abstract
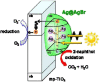
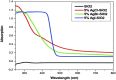
Over the last decade, interest in the utilization of solar energy for photocatalysis treatment processes has taken centre-stage. Researchers had focused on doping TiO2 with SiO2 to obtain an efficient degradation rate of various types of target pollutants both under UV and visible-light irradiation. In order to further improve this degradation effect, some researchers resorted to incorporate plasmonic metal nanoparticles such as silver and gold into the combined TiO2-SiO2 to fully optimize the TiO2-SiO2's potential in the visible-light region. This article focuses on the challenges in utilizing TiO2 in the visible-light region, the contribution of SiO2 in enhancing photocatalytic activities of the TiO2-SiO2 photocatalyst, and the ability of plasmonic metal nanoparticles (Ag and Au) to edge the TiO2-SiO2 photocatalyst toward an efficient solar photocatalyst.
Keywords: TiO2-SiO2; dye; photocatalysis; plasmonic metal nanoparticles; visible region.
Copyright © 2021 Joseph, Taufiq-Yap, Musta, Sarjadi and Elilarasi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Abdullah H., Kuo D. H., Lee J. Y., Wu C. M. (2016). Recyclability of thin nylon film-supported p-CuBiS2/n-TiO2 heterojunction-based nanocomposites for visible light photocatalytic degradation of organic dye. Appl. Phys. A. 122 (8), 750 10.1007/s00339-016-0276-4 - DOI
-
- Abdullah H., Kuo D. H. (2015). Photocatalytic performance of Ag and CuBiS2 nanoparticle-coated SiO2@ TiO2 composite sphere under visible and ultraviolet light irradiation for azo dye degradation with the assistance of numerous nano p–n diodes. J. Phys. Chem. C. 119 (24), 13632–13641. 10.1021/acs.jpcc.5b01970 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

