Schistosoma japonicum Infection in Treg-Specific USP21 Knockout Mice
- PMID: 33628844
- PMCID: PMC7886505
- DOI: 10.1155/2021/6613162
Schistosoma japonicum Infection in Treg-Specific USP21 Knockout Mice
Abstract
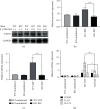
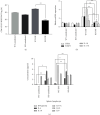
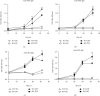
The E3 deubiquitinating enzyme ubiquitin-specific proteolytic enzyme 21 (USP21) plays vital roles in physiological activities and is required for Treg-cell-mediated immune tolerance. Using a murine model infected with Schistosoma japonicum, we observed that there were more cercariae developed into adults and more eggs deposited in the livers of the USP21fl/flFOXP3Cre (KO) mice. However, immunohistochemistry showed that the degree of egg granuloma formation and liver fibrosis was reduced. In USP21fl/flFOXP3Cre mice, levels of IFN-gamma, IL-4, anti-soluble egg antigen (SEA) IgG and anti-soluble worm antigen preparation (SWAP) IgG increased in blood, as determined using ELISAs and multiplex fluorescent microsphere immunoassays, while the levels of IL-10, lL-17A, IL-23, IL-9, and anti-SEA IgM decreased. In addition, the levels of the USP21 protein and mRNA in the liver and spleen of KO mice decreased. We further observed increased Th1 responses amplified by Tregs (regulatory T cells) and compromised Th17 responses, which alleviated the liver immunopathology. We speculated that these changes were related to polarization of Th1-like Tregs. Our results revealed the roles of USP21 in Treg-cell-mediated regulation of immune interactions between Schistosoma and its host. USP21 may have potential for regulating hepatic fibrosis in patients with schistosomiasis.
Copyright © 2021 Youxiang Zhang et al.
Conflict of interest statement
The authors declare that no conflicts of interest exist regarding the publication of this paper.
Figures




Similar articles
-
Insights Into the Properties, Biological Functions, and Regulation of USP21.Front Pharmacol. 2022 Jun 30;13:944089. doi: 10.3389/fphar.2022.944089. eCollection 2022. Front Pharmacol. 2022. PMID: 35846989 Free PMC article. Review.
-
[Imbalance of Th17/Tregs promotes egg granuloma formation of liver with Schistosomiasis japonicum in mice].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2022 Oct;38(10):872-879. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2022. PMID: 36163617 Chinese.
-
T cell-mediated immunity in CBA mice during Schistosoma japonicum infection.Exp Parasitol. 2019 Sep;204:107725. doi: 10.1016/j.exppara.2019.107725. Epub 2019 Jul 12. Exp Parasitol. 2019. PMID: 31306646
-
PPAR-γ Agonist Alleviates Liver and Spleen Pathology via Inducing Treg Cells during Schistosoma japonicum Infection.J Immunol Res. 2018 Jul 17;2018:6398078. doi: 10.1155/2018/6398078. eCollection 2018. J Immunol Res. 2018. PMID: 30116754 Free PMC article.
-
T Lymphocyte-Mediated Liver Immunopathology of Schistosomiasis.Front Immunol. 2020 Feb 18;11:61. doi: 10.3389/fimmu.2020.00061. eCollection 2020. Front Immunol. 2020. PMID: 32132991 Free PMC article. Review.
Cited by
-
Insights Into the Properties, Biological Functions, and Regulation of USP21.Front Pharmacol. 2022 Jun 30;13:944089. doi: 10.3389/fphar.2022.944089. eCollection 2022. Front Pharmacol. 2022. PMID: 35846989 Free PMC article. Review.
-
The ubiquitin-proteasome system in the tumor immune microenvironment: a key force in combination therapy.Front Immunol. 2024 Sep 9;15:1436174. doi: 10.3389/fimmu.2024.1436174. eCollection 2024. Front Immunol. 2024. PMID: 39315102 Free PMC article. Review.
-
Blocking BAFF Alleviates Hepatic Fibrosis in Schistosoma japonicum-Infected Mice.Pathogens. 2023 Jun 1;12(6):793. doi: 10.3390/pathogens12060793. Pathogens. 2023. PMID: 37375483 Free PMC article.
-
IL-33/ST2 Axis Deficiency Exacerbates Hepatic Pathology by Regulating Treg and Th17 Cells in Murine Schistosomiasis Japonica.J Inflamm Res. 2021 Nov 15;14:5981-5998. doi: 10.2147/JIR.S336404. eCollection 2021. J Inflamm Res. 2021. PMID: 34815688 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

