Patient-Reported Outcomes and Long-Term Nonadherence to Aromatase Inhibitors
- PMID: 33629114
- PMCID: PMC8328987
- DOI: 10.1093/jnci/djab022
Patient-Reported Outcomes and Long-Term Nonadherence to Aromatase Inhibitors
Abstract
Background: Nonadherence to aromatase inhibitors (AIs) is common and increases risk of breast cancer (BC) recurrence. We analyzed factors associated with nonadherence among patients enrolled in S1105, a randomized trial of text messaging.
Methods: At enrollment, patients were required to have been on an adjuvant AI for at least 30 days and were asked about financial, medication, and demographic factors. They completed patient-reported outcomes (PROs) representing pain (Brief Pain Inventory), endocrine symptoms (Functional Assessment of Cancer Therapy-Endocrine Symptoms), and beliefs about medications (Treatment Satisfaction Questionnaire for Medicine; Brief Medication Questionnaire). Our primary endpoint was AI nonadherence at 36 months, defined as urine AI metabolite assay of less than 10 ng/mL or no submitted specimen. We evaluated the association between individual baseline characteristics and nonadherence with logistic regression. A composite risk score reflecting the number of statistically significant baseline characteristics was examined.
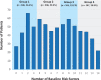
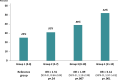
Results: We analyzed data from 702 patients; median age was 60.9 years. Overall, 35.9% patients were nonadherent at 36 months. Younger patients (younger than age 65 years) were more nonadherent (38.8% vs 28.6%, odds ratio [OR] = 1.51, 95% confidence interval [CI] = 1.05 to 2.16; P = .02). Fourteen baseline PRO scales were each statistically significantly associated with nonadherence. In a composite risk model categorized into quartile levels, each increase in risk level was associated with a 46.5% increase in the odds of nonadherence (OR = 1.47, 95% CI =1.26 to 1.70; P < .001). The highest-risk patients were more than 3 times more likely to be nonadherent than the lowest-risk patients (OR = 3.14, 95% CI = 1.97 to 5.02; P < .001).
Conclusions: The presence of multiple baseline PRO-specified risk factors was statistically significantly associated with AI nonadherence. The use of these assessments can help identify patients for targeted interventions to improve adherence.
© The Author(s) 2021. Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

Similar articles
-
Perceived barriers to treatment predict adherence to aromatase inhibitors among breast cancer survivors.Cancer. 2017 Jan 1;123(1):169-176. doi: 10.1002/cncr.30318. Epub 2016 Aug 29. Cancer. 2017. PMID: 27570979 Free PMC article.
-
Randomized Trial of Text Messaging to Reduce Early Discontinuation of Adjuvant Aromatase Inhibitor Therapy in Women With Early-Stage Breast Cancer: SWOG S1105.J Clin Oncol. 2020 Jul 1;38(19):2122-2129. doi: 10.1200/JCO.19.02699. Epub 2020 May 5. J Clin Oncol. 2020. PMID: 32369401 Free PMC article. Clinical Trial.
-
Nonadherence to Medications for Chronic Conditions and Nonadherence to Adjuvant Hormonal Therapy in Women With Breast Cancer.JAMA Oncol. 2016 Oct 1;2(10):1326-1332. doi: 10.1001/jamaoncol.2016.1291. JAMA Oncol. 2016. PMID: 27281650
-
Exercise therapies for preventing or treating aromatase inhibitor-induced musculoskeletal symptoms in early breast cancer.Cochrane Database Syst Rev. 2020 Jan 29;1(1):CD012988. doi: 10.1002/14651858.CD012988.pub2. Cochrane Database Syst Rev. 2020. PMID: 31994181 Free PMC article.
-
Efficacy and toxicity of extended aromatase inhibitors after adjuvant aromatase inhibitors-containing therapy for hormone-receptor-positive breast cancer: a literature-based meta-analysis of randomized trials.Breast Cancer Res Treat. 2020 Jan;179(2):275-285. doi: 10.1007/s10549-019-05464-w. Epub 2019 Oct 12. Breast Cancer Res Treat. 2020. PMID: 31606823 Review.
Cited by
-
From Race to Racism and Disparities to Equity: An Actionable Biopsychosocial Approach to Breast Cancer Outcomes.Cancer J. 2023 Nov-Dec 01;29(6):316-322. doi: 10.1097/PPO.0000000000000677. Cancer J. 2023. PMID: 37963365 Free PMC article. Review.
-
Investigation of Factors Affecting Adherence to Adjuvant Hormone Therapy in Early-Stage Breast Cancer Patients: A Comprehensive Systematic Review.J Breast Cancer. 2023 Aug;26(4):309-333. doi: 10.4048/jbc.2023.26.e22. Epub 2023 May 10. J Breast Cancer. 2023. PMID: 37272247 Free PMC article.
-
Quality-of-life outcomes and risk prediction for patients randomized to nivolumab plus ipilimumab vs nivolumab on LungMAP-S1400I.J Natl Cancer Inst. 2023 Apr 11;115(4):437-446. doi: 10.1093/jnci/djad003. J Natl Cancer Inst. 2023. PMID: 36625510 Free PMC article. Clinical Trial.
-
Neighborhood socioeconomic deprivation and patient-reported outcomes in symptom management trials for women with breast cancer.Breast Cancer Res Treat. 2025 Feb;209(3):603-611. doi: 10.1007/s10549-024-07523-3. Epub 2024 Nov 19. Breast Cancer Res Treat. 2025. PMID: 39560823
-
Alternative dosing regimen of exemestane in a randomized presurgical trial: the role of obesity in biomarker modulation.NPJ Breast Cancer. 2024 Jan 18;10(1):7. doi: 10.1038/s41523-024-00616-8. NPJ Breast Cancer. 2024. PMID: 38238336 Free PMC article.
References
-
- Cuzick J, Sestak I, Forbes JF, et al.Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014;383(9922):1041–1048. - PubMed
-
- Hershman DL. Sticking to it: improving outcomes by increasing adherence. J Clin Oncol. 2016;34(21):2440–2442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

