Human-induced pluripotent stem cell-derived cardiomyocytes, 3D cardiac structures, and heart-on-a-chip as tools for drug research
- PMID: 33629131
- PMCID: PMC8245367
- DOI: 10.1007/s00424-021-02536-z
Human-induced pluripotent stem cell-derived cardiomyocytes, 3D cardiac structures, and heart-on-a-chip as tools for drug research
Abstract
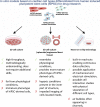
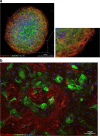
Development of new drugs is of high interest for the field of cardiac and cardiovascular diseases, which are a dominant cause of death worldwide. Before being allowed to be used and distributed, every new potentially therapeutic compound must be strictly validated during preclinical and clinical trials. The preclinical studies usually involve the in vitro and in vivo evaluation. Due to the increasing reporting of discrepancy in drug effects in animal and humans and the requirement to reduce the number of animals used in research, improvement of in vitro models based on human cells is indispensable. Primary cardiac cells are difficult to access and maintain in cell culture for extensive experiments; therefore, the human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) became an excellent alternative. This technology enables a production of high number of patient- and disease-specific cardiomyocytes and other cardiac cell types for a large-scale research. The drug effects can be extensively evaluated in the context of electrophysiological responses with a use of well-established tools, such as multielectrode array (MEA), patch clamp, or calcium ion oscillation measurements. Cardiotoxicity, which is a common reason for withdrawing drugs from marketing or rejection at final stages of clinical trials, can be easily verified with a use of hiPSC-CM model providing a prediction of human-specific responses and higher safety of clinical trials involving patient cohort. Abovementioned studies can be performed using two-dimensional cell culture providing a high-throughput and relatively lower costs. On the other hand, more complex structures, such as engineered heart tissue, organoids, or spheroids, frequently applied as co-culture systems, represent more physiological conditions and higher maturation rate of hiPSC-derived cells. Furthermore, heart-on-a-chip technology has recently become an increasingly popular tool, as it implements controllable culture conditions, application of various stimulations and continuous parameters read-out. This paper is an overview of possible use of cardiomyocytes and other cardiac cell types derived from hiPSC as in vitro models of heart in drug research area prepared on the basis of latest scientific reports and providing thorough discussion regarding their advantages and limitations.
Keywords: 3D structures; Body-on-a-chip; Cardiomyocytes; Cardiotoxicity; Drug research; EHT; Engineered heart tissue; Heart-on-a-chip; Organoids; Spheroids; Stem cells; hiPSC; hiPSC-CMs.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Abadi PPSS, Garbern JC, Behzadi S, Hill MJ, Tresback JS, Heydari T, Ejtehadi MR, Ahmed N, Copley E, Aghaverdi H, Lee RT, Farokhzad OC, Mahmoudi M. Engineering of Mature human induced pluripotent stem cell-derived cardiomyocytes using substrates with multiscale topography. Advanced Functional Materials. 2018;28:1707378. doi: 10.1002/adfm.201707378. - DOI
-
- Abi-Gerges N, Pointon A, Oldman KL, Brown MR, Pilling MA, Sefton CE, Garside H, Pollard CE. Assessment of extracellular field potential and Ca2+ transient signals for early QT/pro-arrhythmia detection using human induced pluripotent stem cell-derived cardiomyocytes. J Pharmacol Toxicol Methods. 2017;83:1–15. doi: 10.1016/j.vascn.2016.09.001. - DOI - PubMed
-
- Abilez OJ, Tzatzalos E, Yang H, Zhao M-T, Jung G, Zöllner AM, Tiburcy M, Riegler J, Matsa E, Shukla P, Zhuge Y, Chour T, Chen VC, Burridge PW, Karakikes I, Kuhl E, Bernstein D, Couture LA, Gold JD, Zimmermann WH, Wu JC. Passive stretch induces structural and functional maturation of engineered heart muscle as predicted by computational modeling. Stem Cells. 2018;36:265–277. doi: 10.1002/stem.2732. - DOI - PMC - PubMed
-
- Acimovic I, Refaat MM, Moreau A, Salykin A, Reiken S, Sleiman Y, Souidi M, Přibyl J, Kajava AV, Richard S, Lu JT, Chevalier P, Skládal P, Dvořak P, Rotrekl V, Marks AR, Scheinman MM, Lacampagne A, Meli AC (2018) Post-translational modifications and diastolic calcium leak associated to the novel RyR2-D3638A mutation lead to CPVT in patient-specific hiPSC-derived cardiomyocytes. J Clin Med 7. 10.3390/jcm7110423 - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

