Distinct and dementia-related synaptopathy in the hippocampus after military blast exposures
- PMID: 33629462
- PMCID: PMC8412116
- DOI: 10.1111/bpa.12936
Distinct and dementia-related synaptopathy in the hippocampus after military blast exposures
Abstract
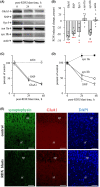
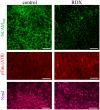
Explosive shockwaves, and other types of blast exposures, are linked to injuries commonly associated with military service and to an increased risk for the onset of dementia. Neurological complications following a blast injury, including depression, anxiety, and memory problems, often persist even when brain damage is undetectable. Here, hippocampal explants were exposed to the explosive 1,3,5-trinitro-1,3,5-triazinane (RDX) to identify indicators of blast-induced changes within important neuronal circuitries. Highly controlled detonations of small, 1.7-gram RDX spherical charges reduced synaptic markers known to be downregulated in cognitive disorders, but without causing overt neuronal loss or astroglial responses. In the absence of neuromorphological alterations, levels of synaptophysin, GluA1, and synapsin IIb were significantly diminished within 24 hr, and these synaptic components exhibited progressive reductions following blast exposure as compared to their stable maintenance in control explants. In contrast, labeling of the synapsin IIa isoform remained unaltered, while neuropilar staining of other markers decreased, including synapsin IIb and neural cell adhesion molecule (NCAM) isoforms, along with evidence of NCAM proteolytic breakdown. NCAM180 displayed a distinct decline after the RDX blasts, whereas NCAM140 and NCAM120 exhibited smaller or no deterioration, respectively. Interestingly, the extent of synaptic marker reduction correlated with AT8-positive tau levels, with tau pathology stochastically found in CA1 neurons and their dendrites. The decline in synaptic components was also reflected in the size of evoked postsynaptic currents recorded from CA1 pyramidals, which exhibited a severe and selective reduction. The identified indicators of blast-mediated synaptopathy point to the need for early biomarkers of explosives altering synaptic integrity with links to dementia risk, to advance strategies for both cognitive health and therapeutic monitoring.
Keywords: Alzheimer-type synaptopathogenesis; NBDP; NCAM breakdown products; mild traumatic brain injury; neurotrauma; synaptic decline.
© 2021 The Authors. Brain Pathology published by John Wiley & Sons Ltd on behalf of International Society of Neuropathology.
Conflict of interest statement
The authors declare that they have no competing interests. The funding agencies had no role in study design, data collection and analysis, or decision to publish.
Figures



Similar articles
-
Blast waves from detonated military explosive reduce GluR1 and synaptophysin levels in hippocampal slice cultures.Exp Neurol. 2016 Dec;286:107-115. doi: 10.1016/j.expneurol.2016.10.002. Epub 2016 Oct 5. Exp Neurol. 2016. PMID: 27720798 Free PMC article.
-
Military blast-induced synaptic changes with distinct vulnerability may explain behavioral alterations in the absence of obvious brain damage.J Nat Sci. 2017 Jul;3(7):e406. J Nat Sci. 2017. PMID: 28824962 Free PMC article.
-
Structural and biochemical abnormalities in the absence of acute deficits in mild primary blast-induced head trauma.J Neurosurg. 2016 Mar;124(3):675-86. doi: 10.3171/2015.1.JNS141571. Epub 2015 Aug 21. J Neurosurg. 2016. PMID: 26295915 Free PMC article.
-
[Mild traumatic brain injury and postconcussive syndrome: a re-emergent questioning].Encephale. 2012 Sep;38(4):329-35. doi: 10.1016/j.encep.2011.07.003. Epub 2011 Aug 31. Encephale. 2012. PMID: 22980474 Review. French.
-
Bomb blast, mild traumatic brain injury and psychiatric morbidity: a review.Injury. 2010 May;41(5):437-43. doi: 10.1016/j.injury.2009.11.018. Epub 2010 Feb 26. Injury. 2010. PMID: 20189170 Review.
Cited by
-
Excitotoxic stimulation activates distinct pathogenic and protective expression signatures in the hippocampus.J Cell Mol Med. 2021 Sep;25(18):9011-9027. doi: 10.1111/jcmm.16864. Epub 2021 Aug 20. J Cell Mol Med. 2021. PMID: 34414662 Free PMC article.
-
Clinical complications after a traumatic brain injury and its relation with brain biomarkers.Sci Rep. 2023 Nov 16;13(1):20057. doi: 10.1038/s41598-023-47267-6. Sci Rep. 2023. PMID: 37973882 Free PMC article.
-
Neuroprotective potential of intranasally delivered L-myc immortalized human neural stem cells in female rats after a controlled cortical impact injury.Sci Rep. 2023 Oct 19;13(1):17874. doi: 10.1038/s41598-023-44426-7. Sci Rep. 2023. PMID: 37857701 Free PMC article.
-
Exercise plasma improves traumatic brain injury outcomes in mice.Sci Rep. 2025 May 23;15(1):17957. doi: 10.1038/s41598-025-02509-7. Sci Rep. 2025. PMID: 40410204 Free PMC article.
-
Viral-mediated increased hippocampal neurogranin modulate synapses at one month in a rat model of controlled cortical impact.Sci Rep. 2024 Nov 22;14(1):28998. doi: 10.1038/s41598-024-77682-2. Sci Rep. 2024. PMID: 39578516 Free PMC article.
References
-
- Brix KA, Brody DL, Grimes JB, Yitzhak A. Working Group Members . Military blast exposure and chronic neurodegeneration: summary of working groups and expert panel findings and recommendations. J Neurotrauma 2017;34(S1):S18–25.
-
- Department of Defense (DoD) . Data from “Worldwide numbers for traumatic brain injury”. https://dvbic.dcoe.mil/dod‐worldwide‐numbers‐tbi. Accessed May 15, 2020.
-
- Williamson V, Mulhall E. Invisible wounds: psychological and neurological injuries confront a new generation of veterans. Iraq and Afghanistan Veterans of America, Issue Report; 2009.
-
- Sawyer TW, Ritzel DV, Wang Y, Josey T, Villanueva M, Nelson P, et al. Primary blast causes delayed effects without cell death in shell‐encased brain cell aggregates. J Neurotrauma. 2018;35:174–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

