GPR126 regulates colorectal cancer cell proliferation by mediating HDAC2 and GLI2 expression
- PMID: 33629464
- PMCID: PMC8088945
- DOI: 10.1111/cas.14868
GPR126 regulates colorectal cancer cell proliferation by mediating HDAC2 and GLI2 expression
Abstract
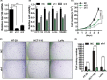
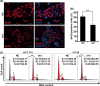
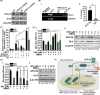
The G-protein-coupled receptor 126 (GPR126) may play an important role in tumor development, although its role remains poorly understood. We found that GPR126 had higher expression in most colorectal cancer cell lines than in normal colon epithelial cell lines, and higher expression levels in colorectal cancer tissues than in normal adjacent colon tissues. GPR126 knockdown induced by shRNA inhibited cell viability and colony formation in HT-29, HCT116, and LoVo cells, decreased BrdU incorporation into newly synthesized proliferating HT-29 cells, led to an arrest of cell cycle progression at the G1 phase in HCT-116 and HT-29 cells, and suppressed tumorigenesis of HT-29, HCT116, and LoVo cells in nude mouse xenograft models. GPR126 knockdown engendered decreased transcription and translation of histone deacetylase 2 (HDAC2), previously implicated in the activation of GLI1 and GLI2 in the Hedgehog signaling pathway. Ectopic expression of HDAC2 in GPR126-silenced cells restored cell viability and proliferation, GLI2 luciferase reporter activity, partially recovered GLI2 expression, and reduced the cell cycle arrest. HDAC2 regulated GLI2 expression and, along with GLI2, it bound to the PTCH1 promoter, as evidenced by a chip assay with HT-29 cells. Purmorphamine, a hedgehog agonist, largely restored the cell viability and expression of GLI2 proteins in GPR126-silenced HT-29 cells, whereas GANT61, a hedgehog inhibitor, further enhanced the GPR126 knockdown-induced inhibitory effects. Our findings demonstrate that GPR126 regulates colorectal cancer cell proliferation by mediating the expression of HDAC2 and GLI2, therefore it may represent a suitable therapeutic target for colorectal cancer treatment.
Keywords: GPR126; RNA-Seq; cell proliferation; colorectal cancer; xenografts.
© 2021 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors have no conflict of interest.
Figures




Similar articles
-
Hedgehog signaling drives cellular survival in human colon carcinoma cells.Cancer Res. 2011 Feb 1;71(3):1092-102. doi: 10.1158/0008-5472.CAN-10-2315. Epub 2010 Dec 6. Cancer Res. 2011. PMID: 21135115 Free PMC article.
-
Role of GLI2 in the growth of human osteosarcoma.J Pathol. 2011 Jun;224(2):169-79. doi: 10.1002/path.2880. Epub 2011 Apr 19. J Pathol. 2011. PMID: 21506130
-
Regulation of DNA damage following termination of Hedgehog (HH) survival signaling at the level of the GLI genes in human colon cancer.Oncotarget. 2012 Aug;3(8):854-68. doi: 10.18632/oncotarget.586. Epub 2012 Aug 20. Oncotarget. 2012. PMID: 23097684 Free PMC article.
-
The Hedgehog signaling pathway promotes chemotherapy resistance via multidrug resistance protein 1 in ovarian cancer.Oncol Rep. 2020 Dec;44(6):2610-2620. doi: 10.3892/or.2020.7798. Epub 2020 Oct 9. Oncol Rep. 2020. PMID: 33125122 Free PMC article.
-
Structure, ligands, and roles of GPR126/ADGRG6 in the development and diseases.Genes Dis. 2023 Mar 27;11(1):294-305. doi: 10.1016/j.gendis.2023.02.016. eCollection 2024 Jan. Genes Dis. 2023. PMID: 37588228 Free PMC article. Review.
Cited by
-
Inhibition of ADAM9 promotes the selective degradation of KRAS and sensitizes pancreatic cancers to chemotherapy.Nat Cancer. 2024 Mar;5(3):400-419. doi: 10.1038/s43018-023-00720-x. Epub 2024 Jan 24. Nat Cancer. 2024. PMID: 38267627
-
Detection of the ADGRG6 hotspot mutations in urine for bladder cancer early screening by ARMS-qPCR.Cancer Med. 2023 May;12(10):11503-11512. doi: 10.1002/cam4.5879. Epub 2023 Apr 20. Cancer Med. 2023. PMID: 37081791 Free PMC article.
-
Molecular characteristics and cancer immunity of LRP1B and its relationship with the Hedgehog signaling pathway in colorectal cancer.Front Immunol. 2025 Mar 18;16:1567102. doi: 10.3389/fimmu.2025.1567102. eCollection 2025. Front Immunol. 2025. PMID: 40170839 Free PMC article.
-
Progesterone activates GPR126 to promote breast cancer development via the Gi pathway.Proc Natl Acad Sci U S A. 2022 Apr 12;119(15):e2117004119. doi: 10.1073/pnas.2117004119. Epub 2022 Apr 8. Proc Natl Acad Sci U S A. 2022. PMID: 35394864 Free PMC article.
-
A novel variant in NBAS identified from an infant with fever-triggered recurrent acute liver failure disrupts the function of the gene.Hum Genome Var. 2023 Apr 13;10(1):13. doi: 10.1038/s41439-023-00241-0. Hum Genome Var. 2023. PMID: 37055399 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- MS12020001/Science Fund for Research Initiation for Doctor of Second Affiliated Hospital of Nantong University, grants from the Nantong Municipal Bureau of Science and Technology
- 81874140/National Natural Science Foundation of China
- 81873555/National Natural Science Foundation of China
- M2020010/Nantong Municipal Health and Family planning commission
- 201740141/Shanghai Health and Family planning commission
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

