Microbial succession and dynamics in meromictic Mono Lake, California
- PMID: 33629529
- PMCID: PMC8359280
- DOI: 10.1111/gbi.12437
Microbial succession and dynamics in meromictic Mono Lake, California
Abstract
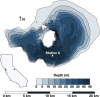
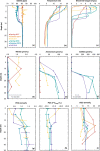
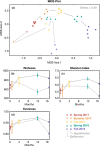
Mono Lake is a closed-basin, hypersaline, alkaline lake located in Eastern Sierra Nevada, California, that is dominated by microbial life. This unique ecosystem offers a natural laboratory for probing microbial community responses to environmental change. In 2017, a heavy snowpack and subsequent runoff led Mono Lake to transition from annually mixed (monomictic) to indefinitely stratified (meromictic). We followed microbial succession during this limnological shift, establishing a two-year (2017-2018) water-column time series of geochemical and microbiological data. Following meromictic conditions, anoxia persisted below the chemocline and reduced compounds such as sulfide and ammonium increased in concentration from near 0 to ~400 and ~150 µM, respectively, throughout 2018. We observed significant microbial succession, with trends varying by water depth. In the epilimnion (above the chemocline), aerobic heterotrophs were displaced by phototrophic genera when a large bloom of cyanobacteria appeared in fall 2018. Bacteria in the hypolimnion (below the chemocline) had a delayed, but systematic, response reflecting colonization by sediment "seed bank" communities. Phototrophic sulfide-oxidizing bacteria appeared first in summer 2017, followed by microbes associated with anaerobic fermentation in spring 2018, and eventually sulfate-reducing taxa by fall 2018. This slow shift indicated that multi-year meromixis was required to establish a sulfate-reducing community in Mono Lake, although sulfide oxidizers thrive throughout mixing regimes. The abundant green alga Picocystis remained the dominant primary producer during the meromixis event, abundant throughout the water column including in the hypolimnion despite the absence of light and prevalence of sulfide. Our study adds to the growing literature describing microbial resistance and resilience during lake mixing events related to climatic events and environmental change.
Keywords: environmental microbiology; geochemistry; limnology; microbial ecology; microbial succession.
© 2021 The Authors. Geobiology published by John Wiley & Sons Ltd.
Figures





References
-
- Armstrong, F. A. J., Stearns, C. R., & Strickland, J. D. H. (1967). The measurement of upwelling and subsequent biological process by means of the Technicon AutoAnalyzer and associated equipment. Deep Sea and Oceanographic Abstracts, 14, 381–390. 10.1016/0011-7471(67)90082-4 - DOI
-
- Arvola, L., George, G., Livingstone, D. M., Järvinen, M., Blenckner, T., Dokulil, M. T., Jennings, E., Aonghusa, C. N., Noges, P., Noges, T., & Weyhenmeyer, G. A. (2010). The impact of the changing climate on the thermal characteristics of lakes. The impact of climate change on European lakes (1st ed., pp. 85–101). Springer.
-
- Benson, L. V., Lund, S. P., Burdett, J. W., Kashgarian, M., Rose, T. P., Smoot, J. P., & Schwartz, M. (1998). Correlation of late‐Pleistocene lake‐level oscillations in Mono Lake, California, with North Atlantic climate events. Quaternary Research, 49, 1–10. 10.1006/qres.1997.1940 - DOI
-
- Bernard, C., Escalas, A., Villeriot, N., Agogue, H., Hugoni, M., Duval, C., Carre, C., Got, P., Sarazin, G., Jezequel, D., Leboulanger, C., Grossi, V., Ader, M., & Troussellier, M. (2019). Very low phytoplankton diversity in a tropical saline‐alkaline lake, with co‐dominance of Arthrospira fusiformis (Cyanobacteria) and Picocystis salinarum (Chlorophyta). Microbial Ecology, 78, 603–617. 10.1007/s00248-019-01332-8 - DOI - PMC - PubMed
-
- Bernhardt, H., & Wilhelms, A. (1967). The continuous determination of low level iron, soluble phosphate and total phosphate with the AutoAnalyzer. Technicon Symposium, 1, 385–389.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

