Key Principles of Clinical Validation, Device Approval, and Insurance Coverage Decisions of Artificial Intelligence
- PMID: 33629545
- PMCID: PMC7909857
- DOI: 10.3348/kjr.2021.0048
Key Principles of Clinical Validation, Device Approval, and Insurance Coverage Decisions of Artificial Intelligence
Abstract
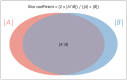
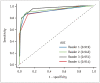
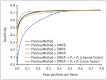
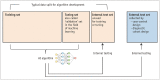
Artificial intelligence (AI) will likely affect various fields of medicine. This article aims to explain the fundamental principles of clinical validation, device approval, and insurance coverage decisions of AI algorithms for medical diagnosis and prediction. Discrimination accuracy of AI algorithms is often evaluated with the Dice similarity coefficient, sensitivity, specificity, and traditional or free-response receiver operating characteristic curves. Calibration accuracy should also be assessed, especially for algorithms that provide probabilities to users. As current AI algorithms have limited generalizability to real-world practice, clinical validation of AI should put it to proper external testing and assisting roles. External testing could adopt diagnostic case-control or diagnostic cohort designs. A diagnostic case-control study evaluates the technical validity/accuracy of AI while the latter tests the clinical validity/accuracy of AI in samples representing target patients in real-world clinical scenarios. Ultimate clinical validation of AI requires evaluations of its impact on patient outcomes, referred to as clinical utility, and for which randomized clinical trials are ideal. Device approval of AI is typically granted with proof of technical validity/accuracy and thus does not intend to directly indicate if AI is beneficial for patient care or if it improves patient outcomes. Neither can it categorically address the issue of limited generalizability of AI. After achieving device approval, it is up to medical professionals to determine if the approved AI algorithms are beneficial for real-world patient care. Insurance coverage decisions generally require a demonstration of clinical utility that the use of AI has improved patient outcomes.
Keywords: Artificial intelligence; Device approval; Insurance coverage; Software validation.
Copyright © 2021 The Korean Society of Radiology.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures

References
-
- Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25:44–56. - PubMed
-
- Park SH, Lim TH. Artificial intelligence: guide for healthcare personnel. Seoul: Koonja; 2020.
-
- Choi JS, Han BK, Ko ES, Bae JM, Ko EY, Song SH, et al. Effect of a deep learning framework-based computer-aided diagnosis system on the diagnostic performance of radiologists in differentiating between malignant and benign masses on breast ultrasonography. Korean J Radiol. 2019;20:749–758. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

