Isolation of alkaliphilic calcifying bacteria and their feasibility for enhanced CaCO3 precipitation in bio-based cementitious composites
- PMID: 33629805
- PMCID: PMC8085925
- DOI: 10.1111/1751-7915.13752
Isolation of alkaliphilic calcifying bacteria and their feasibility for enhanced CaCO3 precipitation in bio-based cementitious composites
Abstract
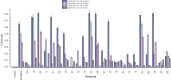
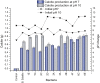
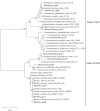
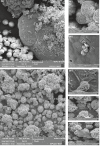
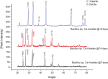
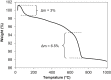
Microbially induced calcite precipitation (MICP), secreted through biological metabolic activity, secured an imperative position in remedial measures within the construction industry subsequent to ecological, environmental and economical returns. However, this contemporary recurrent healing system is susceptible to microbial depletion in the highly alkaline cementitious environment. Therefore, researchers are probing for alkali resistant calcifying microbes. In the present study, alkaliphilic microbes were isolated from different soil sources and screened for probable CaCO3 precipitation. Non-ureolytic pathway (oxidation of organic carbon) was adopted for calcite precipitation to eliminate the production of toxic ammonia. For this purpose, calcium lactate Ca(C3 H5 O3 )2 and calcium acetate Ca(CH3 COO)2 were used as CaCO3 precipitation precursors. The quantification protocol for precipitated CaCO3 was established to select potent microbial species for implementation in the alkaline cementitious systems as more than 50% of isolates were able to precipitate CaCO3 . Results suggested 80% of potent calcifying strains isolated in this study, portrayed higher calcite precipitation at pH 10 when compared to pH 7. Ten superlative morphologically distinct isolates capable of CaCO3 production were identified by 16SrRNA sequencing. Sequenced microbes were identified as species of Bacillus, Arthrobacter, Planococcus, Chryseomicrobium and Corynebacterium. Further, microstructure of precipitated CaCO3 was inspected through scanning electron microscopy (SEM), X-ray diffraction (XRD) and thermal gravimetric (TG) analysis. Then, the selected microbes were investigated in the cementitious mortar to rule out any detrimental effects on mechanical properties. These strains showed maximum of 36% increase in compressive strength and 96% increase in flexural strength. Bacillus, Arthrobacter, Corynebacterium and Planococcus genera have been reported as CaCO3 producers but isolated strains have not yet been investigated in conjunction with cementitious mortar. Moreover, species of Chryseomicrobium and Glutamicibacter were reported first time as calcifying strains.
© 2021 The Authors. Microbial Biotechnology published by Society for Applied Microbiology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures




References
-
- Adjoudj, M. , Ezziane, K. , Kadri, E.H. , Ngo, T.‐T. , and Kaci, A. (2014) Evaluation of rheological parameters of mortar containing various amounts of mineral addition with polycarboxylate superplasticizer. Constr Build Mater 70: 549–559.
-
- Al Omari, M.M.H. , Rashid, I.S. , Qinna, N.A. , Jaber, A.M. , and Badwan, A.A. (2016) Chapter Two ‐ Calcium Carbonate. In Profiles of Drug substances, excipients and related methodology. Brittain, H.G. (ed). Cambridge, MA: Academic Press, pp. 31–132. - PubMed
-
- Al‐Jaroudi, S.S. , Ul‐Hamid, A. , Mohammed, A.R.I. , and Saner, S. (2007) Use of X‐ray powder diffraction for quantitative analysis of carbonate rock reservoir samples. Powder Technol 175: 115–121.
-
- Al‐Thawadi, S.M. (2011) Ureolytic bacteria and calcium carbonate formation as a mechanism of strength enhancement of sand. J Adv Sci Eng Res 1: 98–114.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

