Probing the Influence of Defects, Hydration, and Composition on Prussian Blue Analogues with Pressure
- PMID: 33629831
- PMCID: PMC8028041
- DOI: 10.1021/jacs.0c13181
Probing the Influence of Defects, Hydration, and Composition on Prussian Blue Analogues with Pressure
Abstract
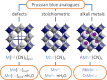
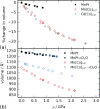
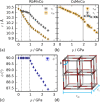
The vast compositional space of Prussian blue analogues (PBAs), formula AxM[M'(CN)6]y·nH2O, allows for a diverse range of functionality. Yet, the interplay between composition and physical properties-e.g., flexibility and propensity for phase transitions-is still largely unknown, despite its fundamental and industrial relevance. Here we use variable-pressure X-ray and neutron diffraction to explore how key structural features, i.e., defects, hydration, and composition, influence the compressibility and phase behavior of PBAs. Defects enhance the flexibility, manifesting as a remarkably low bulk modulus (B0 ≈ 6 GPa) for defective PBAs. Interstitial water increases B0 and enables a pressure-induced phase transition in defective systems. Conversely, hydration does not alter the compressibility of stoichiometric MnPt(CN)6, but changes the high-pressure phase transitions, suggesting an interplay between low-energy distortions. AMnCo(CN)6 (AI = Rb, Cs) transition from F4̅3m to P4̅n2 upon compression due to octahedral tilting, and the critical pressure can be tuned by the A-site cation. At 1 GPa, the symmetry of Rb0.87Mn[Co(CN)6]0.91 is further lowered to the polar space group Pn by an improper ferroelectric mechanism. These fundamental insights aim to facilitate the rational design of PBAs for applications within a wide range of fields.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Yuan A.-H.; Chu C.-X.; Zhou H.; Yuan P.; Liu K.-K.; Li L.; Zhang Q.-F.; Chen X.; Li Y.-Z. Syntheses, crystal structures and gas sorption properties of Prussian blue analogues constructed from [Cr(CN)6]3– building blocks. Eur. J. Inorg. Chem. 2010, 866–871. 10.1002/ejic.200900902. - DOI
-
- Marquez C.; Rivera-Torrente M.; Paalanen P. P.; Weckhuysen B. M.; Cirujano F. G.; De Vos D.; De Baerdemaeker T. Increasing the availability of active sites in Zn-Co double metal cyanides by dispersion onto a SiO2 support. J. Catal. 2017, 354, 92–99. 10.1016/j.jcat.2017.08.008. - DOI
-
- Ludi A.; Güdel H.-U.; Rüegg M. The Structural Chemistry of Prussian Blue Analogs. A Single-Crystal Study of Manganese(II) Hexacyanocobaltate(III), Mn3[Co(CN)6]2·xH2O. Inorg. Chem. 1970, 9, 2224–2227. 10.1021/ic50092a005. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

