Cannabidiol inhibits human glioma by induction of lethal mitophagy through activating TRPV4
- PMID: 33629929
- PMCID: PMC8632311
- DOI: 10.1080/15548627.2021.1885203
Cannabidiol inhibits human glioma by induction of lethal mitophagy through activating TRPV4
Abstract
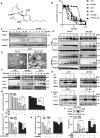
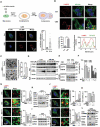
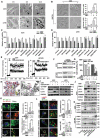
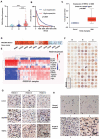
Glioma is the most common primary malignant brain tumor with poor survival and limited therapeutic options. The non-psychoactive phytocannabinoid cannabidiol (CBD) has been shown to be effective against glioma; however, the molecular target and mechanism of action of CBD in glioma are poorly understood. Here we investigated the molecular mechanisms underlying the antitumor effect of CBD in preclinical models of human glioma. Our results showed that CBD induced autophagic rather than apoptotic cell death in glioma cells. We also showed that CBD induced mitochondrial dysfunction and lethal mitophagy arrest, leading to autophagic cell death. Mechanistically, calcium flux induced by CBD through TRPV4 (transient receptor potential cation channel subfamily V member 4) activation played a key role in mitophagy initiation. We further confirmed TRPV4 levels correlated with both tumor grade and poor survival in glioma patients. Transcriptome analysis and other results demonstrated that ER stress and the ATF4-DDIT3-TRIB3-AKT-MTOR axis downstream of TRPV4 were involved in CBD-induced mitophagy in glioma cells. Lastly, CBD and temozolomide combination therapy in patient-derived neurosphere cultures and mouse orthotopic models showed significant synergistic effect in both controlling tumor size and improving survival. Altogether, these findings showed for the first time that the antitumor effect of CBD in glioma is caused by lethal mitophagy and identified TRPV4 as a molecular target and potential biomarker of CBD in glioma. Given the low toxicity and high tolerability of CBD, we therefore propose CBD should be tested clinically for glioma, both alone and in combination with temozolomide.Abbreviations: 4-PBA: 4-phenylbutyrate; AKT: AKT serine/threonine kinase; ATF4: activating transcription factor 4; Baf-A1: bafilomycin A1; CANX: calnexin; CASP3: caspase 3; CAT: catalase; CBD: cannabidiol; CQ: chloroquine; DDIT3: DNA damage inducible transcript 3; ER: endoplasmic reticulum; GBM: glioblastoma multiforme; GFP: green fluorescent protein; MAP1LC3B/LC3B: microtubule associated protein 1 light chain 3 beta; MTOR: mechanistic target of rapamycin kinase; PARP1: poly(ADP-ribose) polymerase; PINK1: PTEN induced kinase 1; PRKN: parkin RBR E3 ubiquitin protein ligase; SLC8A1: solute carrier family 8 member A1; SQSTM1: sequestosome 1; TCGA: The cancer genome atlas; TEM: transmission electron microscopy; TMZ: temozolomide; TRIB3: tribbles pseudokinase 3; TRPC: transient receptor potential cation channel subfamily C; TRPV4: transient receptor potential cation channel subfamily V member 4.
Keywords: Cannabidiol; cannabinoid; glioblastoma; glioma; mitophagy; trpv4.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
