NK/ILC1 cells mediate neuroinflammation and brain pathology following congenital CMV infection
- PMID: 33630019
- PMCID: PMC7918636
- DOI: 10.1084/jem.20201503
NK/ILC1 cells mediate neuroinflammation and brain pathology following congenital CMV infection
Abstract
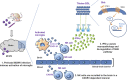
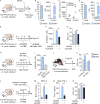
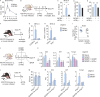
Congenital human cytomegalovirus (cHCMV) infection of the brain is associated with a wide range of neurocognitive sequelae. Using infection of newborn mice with mouse cytomegalovirus (MCMV) as a reliable model that recapitulates many aspects of cHCMV infection, including disseminated infection, CNS infection, altered neurodevelopment, and sensorineural hearing loss, we have previously shown that mitigation of inflammation prevented alterations in cerebellar development, suggesting that host inflammatory factors are key drivers of neurodevelopmental defects. Here, we show that MCMV infection causes a dramatic increase in the expression of the microglia-derived chemokines CXCL9/CXCL10, which recruit NK and ILC1 cells into the brain in a CXCR3-dependent manner. Surprisingly, brain-infiltrating innate immune cells not only were unable to control virus infection in the brain but also orchestrated pathological inflammatory responses, which lead to delays in cerebellar morphogenesis. Our results identify NK and ILC1 cells as the major mediators of immunopathology in response to virus infection in the developing CNS, which can be prevented by anti-IFN-γ antibodies.
© 2021 Kveštak et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures






References
-
- Andrews, S. 2010. FastQC: a quality control tool for high throughput sequence data. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed October 5, 2018).
-
- Blighe, K., Rana S., and Lewis M.. 2020. EnhancedVolcano: Publication-ready volcano plots with enhanced colouring and labeling. R package version 1.6.0. https://github.com/kevinblighe/EnhancedVolcano (accessed November 1, 2019).
-
- Blin, K. 2016. NCBI Genome Downloading Scripts. GitHub Repository. https://github.com/kblin/ncbi-genome-download (accessed September 4, 2019).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

