Ubiquitin-related processes and innate immunity in C. elegans
- PMID: 33630111
- PMCID: PMC11072174
- DOI: 10.1007/s00018-021-03787-w
Ubiquitin-related processes and innate immunity in C. elegans
Abstract
Innate immunity is an evolutionary ancient defence strategy that serves to eliminate infectious agents while maintaining host health. It involves a complex network of sensors, signaling proteins and immune effectors that detect the danger, then relay and execute the immune programme. Post-translational modifications relying on conserved ubiquitin and ubiquitin-like proteins are an integral part of the system. Studies using invertebrate models of infection, such as the nematode Caenorhabditis elegans, have greatly contributed to our understanding of how ubiquitin-related processes act in immune sensing, regulate immune signaling pathways, and participate to host defence responses. This review highlights the interest of working with a genetically tractable model organism and illustrates how C. elegans has been used to identify ubiquitin-dependent immune mechanisms, discover novel ubiquitin-based resistance strategies that mediate pathogen clearance, and unravel the role of ubiquitin-related processes in tolerance, preserving host fitness during pathogen attack. Special emphasis is placed on processes that are conserved in mammals.
Keywords: Host–pathogen interaction; Proteostasis; SUMOylation; Ubiquitination; Unfolded protein response.
Conflict of interest statement
The authors declare that they have no competing interests. All authors contributed to the writing of the manuscript.
Figures
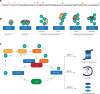
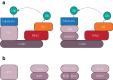
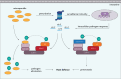
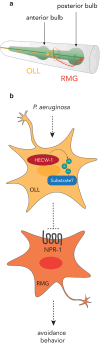
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

