Efficacy and safety of tildrakizumab in Japanese patients with moderate to severe plaque psoriasis: Results from a 64-week phase 3 study (reSURFACE 1)
- PMID: 33630387
- PMCID: PMC8247960
- DOI: 10.1111/1346-8138.15789
Efficacy and safety of tildrakizumab in Japanese patients with moderate to severe plaque psoriasis: Results from a 64-week phase 3 study (reSURFACE 1)
Abstract
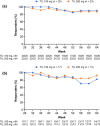
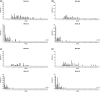
Tildrakizumab is a high-affinity, humanized immunoglobulin G1κ, anti-interleukin-23p19 monoclonal antibody recently approved in Japan for treatment of plaque psoriasis. We report results from Japanese patients treated with tildrakizumab in the multinational, randomized, double-blind, placebo-controlled reSURFACE 1 study (clinicaltrials.gov NCT01722331). Adults with moderate to severe plaque psoriasis were randomized (2:2:1) to receive subcutaneous tildrakizumab 100 or 200 mg or placebo every 12 weeks. Placebo recipients were rerandomized to tildrakizumab 100 or 200 mg at week 12. The global study coprimary endpoints were the proportions of patients achieving 75% improvement from baseline Psoriasis Area and Severity Index (PASI 75) and Physician Global Assessment (PGA) response (0/1 with ≥2 grade reduction from baseline) at week 12. Analyses included 158 Japanese patients randomized to tildrakizumab 100 (n = 64) or 200 mg (n = 62) or placebo (n = 32). Japanese patients had higher mean baseline body surface area involvement and PASI versus all reSURFACE 1 patients. At week 12, significantly more Japanese patients receiving tildrakizumab 100 and 200 mg versus placebo achieved PASI 75 (54.7% and 54.8% vs 6.3%, respectively, both nominal p < 0.001) and PGA 0/1 response (54.7% and 56.5% vs 9.4%, respectively, both nominal P < 0.001). Response rates increased over time with maximal efficacy after 22-28 weeks; >80% of patients achieving PASI 75 or PASI 90 at week 28 and continuing tildrakizumab treatment at the same dose maintained response at week 64. From baseline to week 28, absolute PASI decreased from >12 in all patients to ≤2 in >40% and ≤3 in >50% of patients receiving tildrakizumab. Tildrakizumab was generally well tolerated with an adverse event profile similar to that of placebo. Tildrakizumab treatment was associated with durable efficacy in Japanese patients with moderate to severe plaque psoriasis despite greater baseline disease severity versus the global reSURFACE 1 population.
Keywords: Japanese; antibodies; humanized; interleukin-23 subunit p19; monoclonal; psoriasis; randomized controlled trial; tildrakizumab.
© 2021 The Authors. The Journal of Dermatology published by John Wiley & Sons Australia, Ltd on behalf of Japanese Dermatological Association.
Conflict of interest statement
Dr. Igarashi has received honoraria as a member of an advisory board for AbbVie Inc., Celgene K.K., Eli Lilly Japan K.K., Janssen Pharmaceutical K.K., Maruho Co Ltd., Novartis Pharma K.K., and Sun Pharma Japan Ltd. Dr. Nakagawa received consulting fees and/or speaker honoraria from AbbVie, Janssen Pharmaceutical, Japan Tobacco, Kyowa Kirin, LEO Pharma, Maruho, Torii Pharmaceutical, and UCB Japan. Dr. Morita has received research grants, consulting fees, and/or speaker's fees from AbbVie, Boehringer Ingelheim, Celgene, Eisai, Eli Lilly, Janssen, Kyowa Hakko Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe, Nichi‐Iko, Nippon Kayaku, Novartis Pharmaceuticals, Sun Pharmaceutical Industries, Inc., Taiho Pharmaceutical, and Torii Pharmaceutical, Ushio. Dr. Okubo has received research grants from Eisai, Torii, Maruho, and Shiseido, and has current consulting/advisory board agreements, and/or is on a speaker's bureau and/or is an investigator on clinical trials with AbbVie, Amgen, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Eisai, Eli Lilly, Janssen Pharma, Jimro, Kyowa Kirin, LEO Pharma, Maruho, Novartis Pharma, Pfizer, Sanofi, Sun Pharma, Taiho, Tanabe Mitsubishi, Torii, and UCB Pharma. Dr. Sano has received research grants from AbbVie, Kaken, Kyowa Hakko Kirin, Maruho, Nihon Kayaku, Pola Pharma, Sanofi Aventis, Taiho, and Torii, and speaker's fees from AbbVie, Celgene, Eisai, Eli Lilly, Janssen, Kyowa Hakko Kirin, Maruho, Mitsubishi Tanabe, Novartis Pharma, Sanofi Aventis, Taiho, Torii, and UCB Japan. Dr. Imafuku has received consulting fees and/or honoraria from AbbVie, Eisai, Eli Lilly, Kyowa Kirin, Celgene, Taiho Yakuhin, Tanabe Mitsubishi, Torii Yakuhin, Maruho, LEO pharma, Janssen, and UCB Japan. Dr. Tada has received honoraria for research from Maruho, LEO Pharma, Eisai, AbbVie, Kyowa Hakko Kirin, Celgene, Meiji‐Seika‐pharma, Taiho, Torii, and Eli Lilly & Co., and for lecturing from Maruho, AbbVie, Taiho, Celgene, Mitsubishi Tanabe Pharma Co., Novartis Pharma K.K., UCB, Kyowa Hakko Kirin, LEO Pharma, Eli Lilly & Co., and Janssen Pharmaceutical. Dr. Honma has no conflict of interest to disclose in compliance with the guidance released by the Japanese Dermatology Association. Dr. Mendelsohn is an employee of Sun Pharmaceutical Industries, Inc.; and has individual shares in Johnson and Johnson, and as part of retirement account/mutual funds. Dr. Kawamura is an employee of Sun Pharmaceutical Japan, Ltd. Dr. Ohtsuki has received honoraria as a member of an advisory board for AbbVie Inc., Boehringer Ingelheim Co Ltd., Bristol‐Myers Squibb Company, Celgene K.K., Eisai Co Ltd., Eli Lilly Japan K.K., Janssen Pharmaceutical K.K., Kyowa Hakko Kirin Co Ltd., LEO Pharma K.K., Maruho Co Ltd., Mitsubishi Tanabe Pharma Co., Novartis Pharma K.K., Pfizer Japan Inc., and Sun Pharma Japan Ltd.
Figures

References
-
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377–85. - PubMed
-
- Saeki H, Terui T, Morita A, et al. Japanese guidance for use of biologics for psoriasis (the 2019 version). J Dermatol. 2020;47(3):201–22. - PubMed
-
- Ito T, Takahashi H, Kawada A, Iizuka H, Nakagawa H, Japanese Society For Psoriasis R . Epidemiological survey from 2009 to 2012 of psoriatic patients in Japanese Society for Psoriasis Research. J Dermatol. 2018;45(3):293–301. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

