Practical considerations for optimising homologous recombination repair mutation testing in patients with metastatic prostate cancer
- PMID: 33630412
- PMCID: PMC8185363
- DOI: 10.1002/cjp2.203
Practical considerations for optimising homologous recombination repair mutation testing in patients with metastatic prostate cancer
Abstract
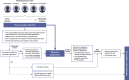
Analysis of the genomic landscape of prostate cancer has identified different molecular subgroups with relevance for novel or existing targeted therapies. The recent approvals of the poly(ADP-ribose) polymerase (PARP) inhibitors olaparib and rucaparib in the metastatic castration-resistant prostate cancer (mCRPC) setting signal the need to embed molecular diagnostics in the clinical pathway of patients with mCRPC to identify those who can benefit from targeted therapies. Best practice guidelines in overall biospecimen collection and processing for molecular analysis are widely available for several tumour types. However, there is no standard protocol for molecular diagnostic testing in prostate cancer. Here, we provide a series of recommendations on specimen handling, sample pre-analytics, laboratory workflow, and testing pathways to maximise the success rates for clinical genomic analysis in prostate cancer. Early involvement of a multidisciplinary team of pathologists, urologists, oncologists, radiologists, nurses, molecular scientists, and laboratory staff is key to enable optimal workflow for specimen selection and preservation at the time of diagnosis so that samples are available for molecular analysis when required. Given the improved outcome of patients with mCRPC and homologous recombination repair gene alterations who have been treated with PARP inhibitors, there is an urgent need to incorporate high-quality genomic testing in the routine clinical pathway of these patients.
Keywords: homologous recombination repair; mCRPC; metastatic prostate cancer; molecular diagnostics; poly(ADP-ribose) polymerase inhibitors.
© 2021 The Authors. The Journal of Pathology: Clinical Research published by The Pathological Society of Great Britain and Ireland & John Wiley & Sons, Ltd.
Figures


References
-
- The Royal College of Surgeons of England. National Prostate Cancer Audit Annual Report 2018. [Accessed September 2020]. Available from: https://www.npca.org.uk/content/uploads/2019/02/NPCA-Annual-Report-2018.pdf
-
- Pommier Y, O'Connor MJ, de Bono J. Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Sci Transl Med 2016; 8: 362ps17. - PubMed
-
- O'Connor MJ. Targeting the DNA damage response in cancer. Mol Cell 2015; 60: 547–560. - PubMed
-
- Blackford AN, Jackson SP. ATM, ATR, and DNA‐PK: the trinity at the heart of the DNA damage response. Mol Cell 2017; 66: 801–817. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

