Deciphering the Chameleonic Chemistry of Allenols: Breaking the Taboo of a Onetime Esoteric Functionality
- PMID: 33630581
- PMCID: PMC8479864
- DOI: 10.1021/acs.chemrev.0c00986
Deciphering the Chameleonic Chemistry of Allenols: Breaking the Taboo of a Onetime Esoteric Functionality
Abstract
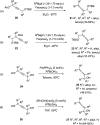
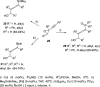
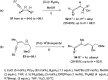
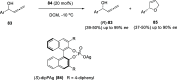
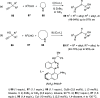
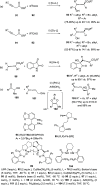
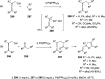
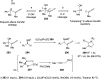
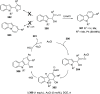
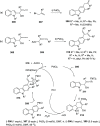
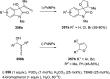
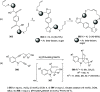
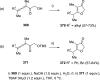
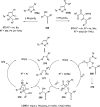
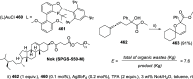
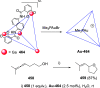
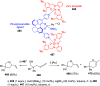
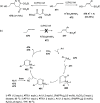
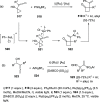
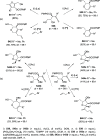
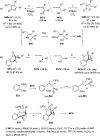
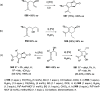
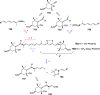
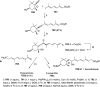
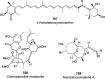
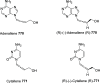
The allene functionality has participated in one of the most exciting voyages in organic chemistry, from chemical curiosities to a recurring building block in modern organic chemistry. In the last decades, a special kind of allene, namely, allenol, has emerged. Allenols, formed by an allene moiety and a hydroxyl functional group with diverse connectivity, have become common building blocks for the synthesis of a wide range of structures and frequent motif in naturally occurring systems. The synergistic effect of the allene and hydroxyl functional groups enables allenols to be considered as a unique and sole functionality exhibiting a special reactivity. This Review summarizes the most significant contributions to the chemistry of allenols that appeared during the past decade, with emphasis on their synthesis, reactivity, and occurrence in natural products.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
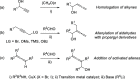

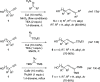
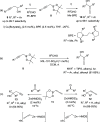
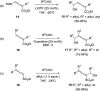



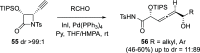


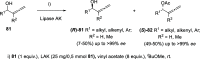






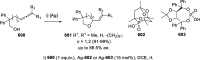
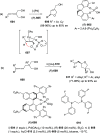
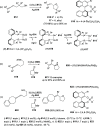
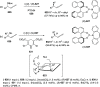



Similar articles
-
Gold-catalyzed cyclization reactions of allenol and alkynol derivatives.Acc Chem Res. 2014 Mar 18;47(3):939-52. doi: 10.1021/ar4002558. Epub 2014 Jan 15. Acc Chem Res. 2014. PMID: 24428670
-
Studies on electrophilic reaction of tertiary 2,3-allenols with NBS in H2O or aqueous MeCN: an efficient selective synthesis of 2-bromoallylic ketones, 1,2-allenyl ketones, or 3-bromo-2,5-dihydrofurans.J Org Chem. 2009 Nov 20;74(22):8733-8. doi: 10.1021/jo9018717. J Org Chem. 2009. PMID: 19873989
-
Fascinating reactivity in gold catalysis: synthesis of oxetenes through rare 4-exo-dig allene cyclization and infrequent β-hydride elimination.Chem Commun (Camb). 2011 Aug 28;47(32):9054-6. doi: 10.1039/c1cc13212a. Epub 2011 Jul 18. Chem Commun (Camb). 2011. PMID: 21766120
-
Creation of Novel Cyclization Methods Using sp-Hybridized Carbon Units and Syntheses of Bioactive Compounds.Chem Pharm Bull (Tokyo). 2017;65(6):511-523. doi: 10.1248/cpb.c17-00088. Chem Pharm Bull (Tokyo). 2017. PMID: 28566644 Review.
-
Exovinylene Cyclic Carbonates: Multifaceted CO2 -Based Building Blocks for Modern Chemistry and Polymer Science.Angew Chem Int Ed Engl. 2022 May 23;61(22):e202116066. doi: 10.1002/anie.202116066. Epub 2022 Apr 5. Angew Chem Int Ed Engl. 2022. PMID: 35266271 Review.
Cited by
-
Iron-Catalyzed Cross-Coupling of Propargyl Ethers with Grignard Reagents for the Synthesis of Functionalized Allenes and Allenols.Angew Chem Int Ed Engl. 2021 Oct 4;60(41):22178-22183. doi: 10.1002/anie.202106742. Epub 2021 Sep 2. Angew Chem Int Ed Engl. 2021. PMID: 34318557 Free PMC article.
-
Ionic Remote α-C-H Allenylation of Silyl Ethers Involving a [1,5]-Hydride Shift Promoted by Silylium-Ion Regeneration.J Am Chem Soc. 2025 Feb 12;147(6):5426-5431. doi: 10.1021/jacs.4c18137. Epub 2025 Jan 31. J Am Chem Soc. 2025. PMID: 39885766 Free PMC article.
-
Iron-Catalyzed Borylation of Propargylic Acetates for the Synthesis of Multisubstituted Allenylboronates.Chemistry. 2023 Jan 12;29(3):e202203130. doi: 10.1002/chem.202203130. Epub 2022 Nov 22. Chemistry. 2023. PMID: 36250587 Free PMC article.
-
Indium-Mediated Preparation of Bis(α-hydroxyallenes) or α,α'-Dihydroxyallenynes and Further Gold-Catalyzed Cyclizations.J Org Chem. 2024 Oct 4;89(19):14228-14232. doi: 10.1021/acs.joc.4c01648. Epub 2024 Sep 17. J Org Chem. 2024. PMID: 39288304 Free PMC article.
-
Stereoselective Synthesis of Allenyl Alcohols by Cobalt(III)-Catalyzed Sequential C-H Bond Addition to 1,3-Enynes and Aldehydes.Angew Chem Int Ed Engl. 2022 Jun 20;61(25):e202202364. doi: 10.1002/anie.202202364. Epub 2022 Apr 29. Angew Chem Int Ed Engl. 2022. PMID: 35420724 Free PMC article.
References
-
- Van’t Hoff J. H.La Chimie dans L’Espace; Bazendijk: Rotterdam, 1875.
-
- Aso M.; Kanematsu K. Allenes as Versatile Synthons. Trends in Organic Chemistry 1995, 5, 157–169.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

