Deficiency of macrophage PHACTR1 impairs efferocytosis and promotes atherosclerotic plaque necrosis
- PMID: 33630758
- PMCID: PMC8262505
- DOI: 10.1172/JCI145275
Deficiency of macrophage PHACTR1 impairs efferocytosis and promotes atherosclerotic plaque necrosis
Abstract
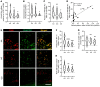
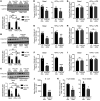
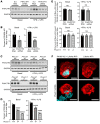
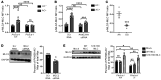
Efferocytosis, the process through which apoptotic cells (ACs) are cleared through actin-mediated engulfment by macrophages, prevents secondary necrosis, suppresses inflammation, and promotes resolution. Impaired efferocytosis drives the formation of clinically dangerous necrotic atherosclerotic plaques, the underlying etiology of coronary artery disease (CAD). An intron of the gene encoding PHACTR1 contains rs9349379 (A>G), a common variant associated with CAD. As PHACTR1 is an actin-binding protein, we reasoned that if the rs9349379 risk allele G causes lower PHACTR1 expression in macrophages, it might link the risk allele to CAD via impaired efferocytosis. We show here that rs9349379-G/G was associated with lower levels of PHACTR1 and impaired efferocytosis in human monocyte-derived macrophages and human atherosclerotic lesional macrophages compared with rs9349379-A/A. Silencing PHACTR1 in human and mouse macrophages compromised AC engulfment, and Western diet-fed Ldlr-/- mice in which hematopoietic Phactr1 was genetically targeted showed impaired lesional efferocytosis, increased plaque necrosis, and thinner fibrous caps - all signs of vulnerable plaques in humans. Mechanistically, PHACTR1 prevented dephosphorylation of myosin light chain (MLC), which was necessary for AC engulfment. In summary, rs9349379-G lowered PHACTR1, which, by lowering phospho-MLC, compromised efferocytosis. Thus, rs9349379-G may contribute to CAD risk, at least in part, by impairing atherosclerotic lesional macrophage efferocytosis.
Keywords: Cardiology; Cardiovascular disease; Cell Biology; Macrophages.
Conflict of interest statement
Figures



Similar articles
-
Resolvin D1 Enhances Necroptotic Cell Clearance Through Promoting Macrophage Fatty Acid Oxidation and Oxidative Phosphorylation.Arterioscler Thromb Vasc Biol. 2021 Mar;41(3):1062-1075. doi: 10.1161/ATVBAHA.120.315758. Epub 2021 Jan 21. Arterioscler Thromb Vasc Biol. 2021. PMID: 33472399 Free PMC article.
-
The Tanyu Tongzhi Formula enhances IL-10 expression and macrophage efferocytosis to alleviate atherosclerosis in mice.Phytomedicine. 2025 Oct;146:157122. doi: 10.1016/j.phymed.2025.157122. Epub 2025 Aug 5. Phytomedicine. 2025. PMID: 40779821
-
Age-Related Impairments in Immune Cell Efferocytosis and Autophagy Hinder Atherosclerosis Regression.Arterioscler Thromb Vasc Biol. 2025 Apr;45(4):481-495. doi: 10.1161/ATVBAHA.124.321662. Epub 2025 Feb 13. Arterioscler Thromb Vasc Biol. 2025. PMID: 39945065 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Sickle Cell Disease.2003 Sep 15 [updated 2025 Feb 13]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. 2003 Sep 15 [updated 2025 Feb 13]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. PMID: 20301551 Free Books & Documents. Review.
Cited by
-
Bioinformatics Identification of Aberrantly Methylated Differentially Expressed Genes Associated with Arteriosclerosis by Integrative Analysis of Gene Expression and DNA Methylation Datasets.Genes (Basel). 2022 Oct 8;13(10):1818. doi: 10.3390/genes13101818. Genes (Basel). 2022. PMID: 36292702 Free PMC article.
-
Mechanistic insights into the regression of atherosclerotic plaques.Front Physiol. 2024 Nov 19;15:1473709. doi: 10.3389/fphys.2024.1473709. eCollection 2024. Front Physiol. 2024. PMID: 39628943 Free PMC article. Review.
-
Developmental endothelial locus 1: the present and future of an endogenous factor in vessels.Front Physiol. 2024 Aug 9;15:1347888. doi: 10.3389/fphys.2024.1347888. eCollection 2024. Front Physiol. 2024. PMID: 39206385 Free PMC article. Review.
-
PECAM1, COL4A2, PHACTR1, and LMOD1 Gene Polymorphisms in Patients with Unstable Angina.J Clin Med. 2022 Jan 13;11(2):373. doi: 10.3390/jcm11020373. J Clin Med. 2022. PMID: 35054067 Free PMC article.
-
Early intermittent hyperlipidaemia alters tissue macrophages to fuel atherosclerosis.Nature. 2024 Oct;634(8033):457-465. doi: 10.1038/s41586-024-07993-x. Epub 2024 Sep 4. Nature. 2024. PMID: 39231480 Free PMC article.
References
-
- Virani SS, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–e596. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- S10 RR027050/RR/NCRR NIH HHS/United States
- R35 HL145228/HL/NHLBI NIH HHS/United States
- R01 HL150359/HL/NHLBI NIH HHS/United States
- R01 HL113147/HL/NHLBI NIH HHS/United States
- T32 HL007343/HL/NHLBI NIH HHS/United States
- TL1 TR001875/TR/NCATS NIH HHS/United States
- P01 HL087123/HL/NHLBI NIH HHS/United States
- UL1 TR001873/TR/NCATS NIH HHS/United States
- R00 HL130574/HL/NHLBI NIH HHS/United States
- R01 HL141127/HL/NHLBI NIH HHS/United States
- P30 CA013696/CA/NCI NIH HHS/United States
- R01 HL151611/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

