First Month of COVID-19 Vaccine Safety Monitoring - United States, December 14, 2020-January 13, 2021
- PMID: 33630816
- PMCID: PMC8344985
- DOI: 10.15585/mmwr.mm7008e3
First Month of COVID-19 Vaccine Safety Monitoring - United States, December 14, 2020-January 13, 2021
Abstract
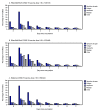
Two coronavirus disease 2019 (COVID-19) vaccines are currently authorized for use in the United States. The Food and Drug Administration (FDA) issued Emergency Use Authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine on December 11, 2020, and for the Moderna COVID-19 vaccine on December 18, 2020; each is administered as a 2-dose series. The Advisory Committee on Immunization Practices issued interim recommendations for Pfizer-BioNTech and Moderna COVID-19 vaccines on December 12, 2020 (1), and December 19, 2020 (2), respectively; initial doses were recommended for health care personnel and long-term care facility (LTCF) residents (3). Safety monitoring for these vaccines has been the most intense and comprehensive in U.S. history, using the Vaccine Adverse Event Reporting System (VAERS), a spontaneous reporting system, and v-safe,* an active surveillance system, during the initial implementation phases of the COVID-19 national vaccination program (4). CDC conducted descriptive analyses of safety data from the first month of vaccination (December 14, 2020-January 13, 2021). During this period, 13,794,904 vaccine doses were administered, and VAERS received and processed† 6,994 reports of adverse events after vaccination, including 6,354 (90.8%) that were classified as nonserious and 640 (9.2%) as serious.§ The symptoms most frequently reported to VAERS were headache (22.4%), fatigue (16.5%), and dizziness (16.5%). A total of 113 deaths were reported to VAERS, including 78 (65%) among LTCF residents; available information from death certificates, autopsy reports, medical records, and clinical descriptions from VAERS reports and health care providers did not suggest any causal relationship between COVID-19 vaccination and death. Rare cases of anaphylaxis after receipt of both vaccines were reported (4.5 reported cases per million doses administered). Among persons who received Pfizer-BioNTech vaccine, reactions reported to the v-safe system were more frequent after receipt of the second dose than after the first. The initial postauthorization safety profiles of the two COVID-19 vaccines in current use did not indicate evidence of unexpected serious adverse events. These data provide reassurance and helpful information regarding what health care providers and vaccine recipients might expect after vaccination.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Geoffrey M. Calvert reports owning 100 shares of Johnson & Johnson stock. No other potential conflicts of interest were disclosed.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

