Pulsed-electromagnetic-field induced osteoblast differentiation requires activation of genes downstream of adenosine receptors A2A and A3
- PMID: 33630907
- PMCID: PMC7906300
- DOI: 10.1371/journal.pone.0247659
Pulsed-electromagnetic-field induced osteoblast differentiation requires activation of genes downstream of adenosine receptors A2A and A3
Abstract
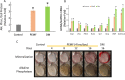
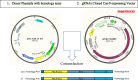
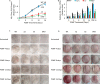
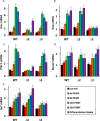
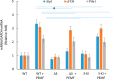
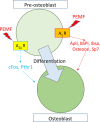
Pulsed-electromagnetic-field (PEMF) treatment was found to enhance cellular differentiation of the mouse preosteoblast, MC3T3-E1, to a more osteoblastic phenotype. Differentiation genes such as Alp, BSPI, cFos, Ibsp, Osteocalcin, Pthr1 and Runx2 showed increased expression in response to PEMF stimulation. Detailed molecular mechanisms linking PEMF to the activation of these genes are limited. Two adenosine receptors known to be modulated in response to PEMF, Adora2A and Adora3, were functionally impaired by CRISPR-Cas9-mediated gene disruption, and the consequences of which were studied in the context of PEMF-mediated osteoblastic differentiation. Disruption of Adora2A resulted in a delay of Alp mRNA expression, but not alkaline phosphatase protein expression, which was similar to that found in wild type cells. However, Adora3 disruption resulted in significantly reduced responses at both the alkaline phosphatase mRNA and protein levels throughout the PEMF stimulation period. Defects observed in response to PEMF were mirrored using a chemically defined growth and differentiation-inducing media (DM). Moreover, in cells with Adora2A disruption, gene expression profiles showed a blunted response in cFos and Pthr1 to PEMF treatment; whereas cells with Adora3 disruption had mostly blunted responses in AlpI, BSPI, Ibsp, Osteocalcin and Sp7 gene activation. To demonstrate specificity for Adora3 function, the Adora3 open reading frame was inserted into the ROSA26 locus in Adora3 disrupted cells culminating in rescued PEMF responsiveness and thereby eliminating the possibility of off-target effects. These results lead us to propose that there are complementary and parallel positive roles for adenosine receptor A2A and A3 in PEMF-mediated osteoblast differentiation.
Conflict of interest statement
The authors have read the journal’s policy and have the following competing interests: NZ is an employee of and own stock in Orthofix Medical Inc. and EIW and JTR are consultants and own stock in Orthofix Medical Inc. (https://www.orthofix.com/). There are no patents, products in development associated with this research to declare. The pulsed electromagnetic signal (PhysioStim®, Orthofix Medical Inc., Lewisville TX) used in this study is FDA-approved for the clinical treatment of long bone non-unions. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

Similar articles
-
Augmentation of Deficient Bone Healing by Pulsed Electromagnetic Fields-From Mechanisms to Clinical Outcomes.Bioengineering (Basel). 2024 Dec 3;11(12):1223. doi: 10.3390/bioengineering11121223. Bioengineering (Basel). 2024. PMID: 39768041 Free PMC article. Review.
-
Pulsed electromagnetic fields increased the anti-inflammatory effect of A₂A and A₃ adenosine receptors in human T/C-28a2 chondrocytes and hFOB 1.19 osteoblasts.PLoS One. 2013 May 31;8(5):e65561. doi: 10.1371/journal.pone.0065561. Print 2013. PLoS One. 2013. PMID: 23741498 Free PMC article.
-
Effects of PEMF exposure at different pulses on osteogenesis of MC3T3-E1 cells.Arch Oral Biol. 2014 Sep;59(9):921-7. doi: 10.1016/j.archoralbio.2014.05.015. Epub 2014 May 28. Arch Oral Biol. 2014. PMID: 24907521
-
Pulsed electromagnetic fields promote the proliferation and differentiation of osteoblasts by reinforcing intracellular calcium transients.Bioelectromagnetics. 2017 Oct;38(7):541-549. doi: 10.1002/bem.22076. Epub 2017 Aug 18. Bioelectromagnetics. 2017. PMID: 28833306
-
Pulsed Electromagnetic Field Stimulation of Bone Healing and Joint Preservation: Cellular Mechanisms of Skeletal Response.J Am Acad Orthop Surg Glob Res Rev. 2020 May;4(5):e1900155. doi: 10.5435/JAAOSGlobal-D-19-00155. J Am Acad Orthop Surg Glob Res Rev. 2020. PMID: 33970582 Free PMC article. Review.
Cited by
-
Pulsed Electromagnetic Field Stimulation in Lumbar Spine Fusion for Patients With Risk Factors for Pseudarthrosis.Int J Spine Surg. 2023 Dec 26;17(6):816-823. doi: 10.14444/8549. Int J Spine Surg. 2023. PMID: 37884337 Free PMC article.
-
Pulsed Electromagnetic Fields for Cervical Spine Fusion in Patients with Risk Factors for Pseudarthrosis.Orthop Rev (Pavia). 2024 Oct 3;16:122534. doi: 10.52965/001c.122534. eCollection 2024. Orthop Rev (Pavia). 2024. PMID: 39698480 Free PMC article.
-
Insights into bone and cartilage responses to pulsed electromagnetic field stimulation: a review with quantitative comparisons.Front Bioeng Biotechnol. 2025 Jul 10;13:1557572. doi: 10.3389/fbioe.2025.1557572. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40708859 Free PMC article. Review.
-
Augmentation of Deficient Bone Healing by Pulsed Electromagnetic Fields-From Mechanisms to Clinical Outcomes.Bioengineering (Basel). 2024 Dec 3;11(12):1223. doi: 10.3390/bioengineering11121223. Bioengineering (Basel). 2024. PMID: 39768041 Free PMC article. Review.
-
Ion channels as molecular targets of glioblastoma electrotherapy.Front Cell Neurosci. 2023 Mar 17;17:1133984. doi: 10.3389/fncel.2023.1133984. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37006466 Free PMC article. Review.
References
-
- Muhsin AU, Islam KM, Ahmed AM, Islam MS, Rabbani KS, Rahman SM, et al.. Effect of pulsed electromagnetic field on healing of experimental nonunion in rat tibiae. Bangladesh Med Res Counc Bull. 1991;17(1):1–10. Epub 1991/06/01. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

