Root hairs are the most important root trait for rhizosheath formation of barley (Hordeum vulgare), maize (Zea mays) and Lotus japonicus (Gifu)
- PMID: 33631013
- PMCID: PMC8318254
- DOI: 10.1093/aob/mcab029
Root hairs are the most important root trait for rhizosheath formation of barley (Hordeum vulgare), maize (Zea mays) and Lotus japonicus (Gifu)
Abstract
Background and aims: Rhizosheaths are defined as the soil adhering to the root system after it is extracted from the ground. Root hairs and mucilage (root exudates) are key root traits involved in rhizosheath formation, but to better understand the mechanisms involved their relative contributions should be distinguished.
Methods: The ability of three species [barley (Hordeum vulgare), maize (Zea mays) and Lotus japonicus (Gifu)] to form a rhizosheath in a sandy loam soil was compared with that of their root-hairless mutants [bald root barley (brb), maize root hairless 3 (rth3) and root hairless 1 (Ljrhl1)]. Root hair traits (length and density) of wild-type (WT) barley and maize were compared along with exudate adhesiveness of both barley and maize genotypes. Furthermore, root hair traits and exudate adhesiveness from different root types (axile versus lateral) were compared within the cereal species.
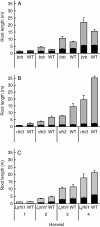
Key results: Per unit root length, rhizosheath size diminished in the order of barley > L. japonicus > maize in WT plants. Root hairs significantly increased rhizosheath formation of all species (3.9-, 3.2- and 1.8-fold for barley, L. japonicus and maize, respectively) but there was no consistent genotypic effect on exudate adhesiveness in the cereals. While brb exudates were more and rth3 exudates were less adhesive than their respective WTs, maize rth3 bound more soil than barley brb. Although both maize genotypes produced significantly more adhesive exudate than the barley genotypes, root hair development of WT barley was more extensive than that of WT maize. Thus, the greater density of longer root hairs in WT barley bound more soil than WT maize. Root type did not seem to affect rhizosheath formation, unless these types differed in root length.
Conclusions: When root hairs were present, greater root hair development better facilitated rhizosheath formation than root exudate adhesiveness. However, when root hairs were absent root exudate adhesiveness was a more dominant trait.
Keywords: L. japonicus; Rhizosheath; barley; maize; root hairs; root mucilage.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Annals of Botany Company.
Figures








Similar articles
-
Altered properties and structures of root exudate polysaccharides in a root hairless mutant of barley.Plant Physiol. 2022 Sep 28;190(2):1214-1227. doi: 10.1093/plphys/kiac341. Plant Physiol. 2022. PMID: 35876808 Free PMC article.
-
Root hair length and rhizosheath mass depend on soil porosity, strength and water content in barley genotypes.Planta. 2014 Mar;239(3):643-51. doi: 10.1007/s00425-013-2002-1. Epub 2013 Dec 8. Planta. 2014. PMID: 24318401
-
Significance of root hairs for plant performance under contrasting field conditions and water deficit.Ann Bot. 2021 Jul 28;128(1):1-16. doi: 10.1093/aob/mcaa181. Ann Bot. 2021. PMID: 33038211 Free PMC article.
-
The role of root hairs in water uptake: recent advances and future perspectives.J Exp Bot. 2022 Jun 2;73(11):3330-3338. doi: 10.1093/jxb/erac114. J Exp Bot. 2022. PMID: 35323893 Review.
-
Rhizosheath: An adaptive root trait to improve plant tolerance to phosphorus and water deficits?Plant Cell Environ. 2022 Oct;45(10):2861-2874. doi: 10.1111/pce.14395. Epub 2022 Jul 25. Plant Cell Environ. 2022. PMID: 35822342 Free PMC article. Review.
Cited by
-
Not so hidden anymore: Advances and challenges in understanding root growth under water deficits.Plant Cell. 2024 May 1;36(5):1377-1409. doi: 10.1093/plcell/koae055. Plant Cell. 2024. PMID: 38382086 Free PMC article. Review.
-
Root phenotypes for improved nitrogen capture.Plant Soil. 2024;502(1-2):31-85. doi: 10.1007/s11104-023-06301-2. Epub 2023 Oct 4. Plant Soil. 2024. PMID: 39323575 Free PMC article. Review.
-
A highly conserved ABCG transporter mediates root-soil cohesion in Arabidopsis.Plant Physiol. 2025 Apr 30;198(1):kiaf193. doi: 10.1093/plphys/kiaf193. Plant Physiol. 2025. PMID: 40350928 Free PMC article.
-
Ensuring future food security and resource sustainability: insights into the rhizosphere.iScience. 2022 Mar 26;25(4):104168. doi: 10.1016/j.isci.2022.104168. eCollection 2022 Apr 15. iScience. 2022. PMID: 35434553 Free PMC article. Review.
-
OsUGE1 is directly targeted by OsGRF6 to regulate root hair length in rice.Theor Appl Genet. 2023 Apr 11;136(5):108. doi: 10.1007/s00122-023-04356-4. Theor Appl Genet. 2023. PMID: 37039968
References
-
- Adu MO, Asare PA, Yawson DO, et al. . 2017. Quantifying variations in rhizosheath and root system phenotypes of landraces and improved varieties of juvenile maize. Rhizosphere 3: 29–39.
-
- Ahmed MA, Zarebanadkouki M, Kaestner A, Carminati A. 2015. Measurements of water uptake of maize roots: the key function of lateral roots. Plant and Soil 398: 59–77.
-
- Albalasmeh AA, Ghezzehei TA. 2013. Interplay between soil drying and root exudation in rhizosheath development. Plant and Soil 374: 739–751.
-
- Bates TR, Lynch JP. 2000. The efficiency of Arabidopsis thaliana (Brassicaceae) root hairs in phosphorus acquisition. American Journal of Botany 87: 964–970. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

