Tocilizumab in Hospitalized Patients with Severe Covid-19 Pneumonia
- PMID: 33631066
- PMCID: PMC7953459
- DOI: 10.1056/NEJMoa2028700
Tocilizumab in Hospitalized Patients with Severe Covid-19 Pneumonia
Abstract
Background: Coronavirus disease 2019 (Covid-19) is associated with immune dysregulation and hyperinflammation, including elevated interleukin-6 levels. The use of tocilizumab, a monoclonal antibody against the interleukin-6 receptor, has resulted in better outcomes in patients with severe Covid-19 pneumonia in case reports and retrospective observational cohort studies. Data are needed from randomized, placebo-controlled trials.
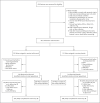
Methods: In this phase 3 trial, we randomly assigned patients who were hospitalized with severe Covid-19 pneumonia in a 2:1 ratio receive a single intravenous infusion of tocilizumab (at a dose of 8 mg per kilogram of body weight) or placebo. Approximately one quarter of the participants received a second dose of tocilizumab or placebo 8 to 24 hours after the first dose. The primary outcome was clinical status at day 28 on an ordinal scale ranging from 1 (discharged or ready for discharge) to 7 (death) in the modified intention-to-treat population, which included all the patients who had received at least one dose of tocilizumab or placebo.
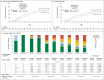
Results: Of the 452 patients who underwent randomization, 438 (294 in the tocilizumab group and 144 in the placebo group) were included in the primary and secondary analyses. The median value for clinical status on the ordinal scale at day 28 was 1.0 (95% confidence interval [CI], 1.0 to 1.0) in the tocilizumab group and 2.0 (non-ICU hospitalization without supplemental oxygen) (95% CI, 1.0 to 4.0) in the placebo group (between-group difference, -1.0; 95% CI, -2.5 to 0; P = 0.31 by the van Elteren test). In the safety population, serious adverse events occurred in 103 of 295 patients (34.9%) in the tocilizumab group and in 55 of 143 patients (38.5%) in the placebo group. Mortality at day 28 was 19.7% in the tocilizumab group and 19.4% in the placebo group (weighted difference, 0.3 percentage points; 95% CI, -7.6 to 8.2; nominal P = 0.94).
Conclusions: In this randomized trial involving hospitalized patients with severe Covid-19 pneumonia, the use of tocilizumab did not result in significantly better clinical status or lower mortality than placebo at 28 days. (Funded by F. Hoffmann-La Roche and the Department of Health and Human Services; COVACTA ClinicalTrials.gov number, NCT04320615.).
Copyright © 2021 Massachusetts Medical Society.
Figures
Comment in
-
Interleukin-6 Receptor Inhibition in Covid-19 - Cooling the Inflammatory Soup.N Engl J Med. 2021 Apr 22;384(16):1564-1565. doi: 10.1056/NEJMe2103108. Epub 2021 Feb 25. N Engl J Med. 2021. PMID: 33631064 Free PMC article. No abstract available.
-
Tocilizumab in COVID-19 therapy: who benefits, and how?Lancet. 2021 Jul 24;398(10297):299-300. doi: 10.1016/S0140-6736(21)01427-6. Lancet. 2021. PMID: 34303430 Free PMC article. No abstract available.
-
Tocilizumab in COVID-19 therapy: who benefits, and how?Lancet. 2021 Jul 24;398(10297):299. doi: 10.1016/S0140-6736(21)01380-5. Lancet. 2021. PMID: 34303431 Free PMC article. No abstract available.
-
Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19.N Engl J Med. 2021 Sep 16;385(12):1147. doi: 10.1056/NEJMc2108482. Epub 2021 Aug 18. N Engl J Med. 2021. PMID: 34407334 No abstract available.
References
-
- World Health Organization. Coronavirus disease (COVID-19) pandemic. 2020. (https://www.who.int/emergencies/diseases/novel-coronavirus-2019).
-
- Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A. SARS-CoV-2, SARS-CoV-1 and MERS-CoV viral load dynamics, duration of viral shedding and infectiousness — a living systematic review and meta-analysis. July 29, 2020. (https://www.medrxiv.org/content/10.1101/2020.07.25.20162107v2). preprint. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
