Seasonal human coronavirus antibodies are boosted upon SARS-CoV-2 infection but not associated with protection
- PMID: 33631096
- PMCID: PMC7871851
- DOI: 10.1016/j.cell.2021.02.010
Seasonal human coronavirus antibodies are boosted upon SARS-CoV-2 infection but not associated with protection
Abstract
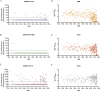
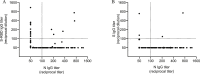
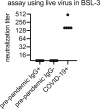
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has rapidly spread within the human population. Although SARS-CoV-2 is a novel coronavirus, most humans had been previously exposed to other antigenically distinct common seasonal human coronaviruses (hCoVs) before the coronavirus disease 2019 (COVID-19) pandemic. Here, we quantified levels of SARS-CoV-2-reactive antibodies and hCoV-reactive antibodies in serum samples collected from 431 humans before the COVID-19 pandemic. We then quantified pre-pandemic antibody levels in serum from a separate cohort of 251 individuals who became PCR-confirmed infected with SARS-CoV-2. Finally, we longitudinally measured hCoV and SARS-CoV-2 antibodies in the serum of hospitalized COVID-19 patients. Our studies indicate that most individuals possessed hCoV-reactive antibodies before the COVID-19 pandemic. We determined that ∼20% of these individuals possessed non-neutralizing antibodies that cross-reacted with SARS-CoV-2 spike and nucleocapsid proteins. These antibodies were not associated with protection against SARS-CoV-2 infections or hospitalizations, but they were boosted upon SARS-CoV-2 infection.
Keywords: SARS-CoV-2; antibodies; coronavirus; pre-existing immunity.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A.C.H. is a consultant for Immunai. E.J.W. has consulting agreements with and/or is on the scientific advisory board for Merck, Elstar, Janssen, Related Sciences, Synthekine, and Surface Oncology. E.J.W. is a founder of Surface Oncology and Arsenal Biosciences. E.J.W. is an inventor on a patent (U.S. patent number 10,370,446) submitted by Emory University that covers the use of PD-1 blockade to treat infections and cancer. D.J.R. is on scientific advisory boards for Alnylam, Metrea, Novartis, Pfizer, and Verve; is a consultant for and receives research support from Regeneron for work unrelated to this report; and is a founder of Vascular Strategies and Staten Biotechnologies. S.E.H. has received consultancy fee from Sanofi Pasteur, Lumen, Novavax, and Merck for work unrelated to this report.
Figures







Update of
-
Seasonal human coronavirus antibodies are boosted upon SARS-CoV-2 infection but not associated with protection.medRxiv [Preprint]. 2020 Nov 10:2020.11.06.20227215. doi: 10.1101/2020.11.06.20227215. medRxiv. 2020. Update in: Cell. 2021 Apr 1;184(7):1858-1864.e10. doi: 10.1016/j.cell.2021.02.010. PMID: 33200143 Free PMC article. Updated. Preprint.
References
-
- Braun J., Loyal L., Frentsch M., Wendisch D., Georg P., Kurth F., Hippenstiel S., Dingeldey M., Kruse B., Fauchere F., et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020;587:270–274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

