Prolonged SARS-CoV-2 cell culture replication in respiratory samples from patients with severe COVID-19
- PMID: 33631334
- PMCID: PMC7898982
- DOI: 10.1016/j.cmi.2021.02.014
Prolonged SARS-CoV-2 cell culture replication in respiratory samples from patients with severe COVID-19
Abstract
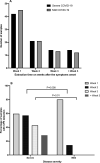
Objectives: This study compares the infectivity of SARS-CoV-2 in respiratory samples from patients with mild COVID-19 with those from hospitalized patients with severe bilateral pneumonia. In severe COVID-19, we also analysed the presence of neutralizing activity in paired sera.
Methods: We performed cell cultures on 193 real-time reverse transcription polymerase chain reaction respiratory samples, positive for SARS-CoV-2, obtained from 189 patients at various times, from clinical diagnosis to follow-up. Eleven samples were obtained from asymptomatic individuals, 91 samples from 91 outpatients with mild forms of COVID-19 and 91 samples from 87 inpatients with severe pneumonia. In these patients, neutralizing activity was analysed in 30 paired sera collected after symptom onset >10 days.
Results: We detected a cytopathic effect (CPE) in 91/193 (47%) samples. Viral viability was maintained for up to 10 days in patients with mild COVID-19. In patients with severe COVID-19, the virus remained viable for up to 32 days after the onset of symptoms. Patients with severe COVID-19 presented infectious virus at a significantly higher rate in the samples with moderate to low viral load (cycle threshold value ≥ 26): 32/75 (43%) versus 14/63 (22%) for mild cases (p < 0.01). We observed a positive CPE despite the presence of clear neutralizing activity (NT50 > 1:1024 in 10% (3/30) of samples.
Discussion: Patients with severe COVID-19 might shed viable virus during prolonged periods of up to 4 weeks after symptom onset, even when presenting high cycle threshold values in their respiratory samples and despite having developed high neutralizing antibody titres.
Keywords: COVID-19; Cell culture; Infection control; Neutralizing antibodies; SARS-CoV-2.
Copyright © 2021 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures


Comment in
-
Duration of SARS-CoV-2 viral culture positivity among different specimen types.Clin Microbiol Infect. 2021 Oct;27(10):1540-1541. doi: 10.1016/j.cmi.2021.07.004. Epub 2021 Jul 16. Clin Microbiol Infect. 2021. PMID: 34274524 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

