Verifying the role of 3-hydroxy of 17-cyclopropylmethyl-4,5α-epoxy-3,14β-dihydroxy-6β-[(4'-pyridyl) carboxamido]morphinan derivatives via their binding affinity and selectivity profiles on opioid receptors
- PMID: 33631465
- PMCID: PMC8009840
- DOI: 10.1016/j.bioorg.2021.104702
Verifying the role of 3-hydroxy of 17-cyclopropylmethyl-4,5α-epoxy-3,14β-dihydroxy-6β-[(4'-pyridyl) carboxamido]morphinan derivatives via their binding affinity and selectivity profiles on opioid receptors
Abstract
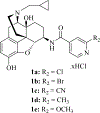
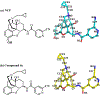
In the present study, the role of 3-hydroxy group of a series of epoxymorphinan derivatives in their binding affinity and selectivity profiles toward the opioid receptors (ORs) has been investigated. It was found that the 3-hydroxy group was crucial for the binding affinity of these derivatives for all three ORs due to the fact that all the analogues 1a-e exhibited significantly higher binding affinities compared to their counterpart 3-dehydroxy ones 6a-e. Meanwhile most compounds carrying the 3-hydroxy group possessed similar selectivity profiles for the kappa opioid receptor over the mu opioid receptor as their corresponding 3-dehydroxy derivatives. [35S]-GTPγS functional assay results indicated that the 3-hydroxy group of these epoxymorphinan derivatives was important for maintaining their potency on the ORs with various effects. Further molecular modeling studies helped comprehend the remarkably different binding affinity and functional profiles between compound 1c (NCP) and its 3-dehydroxy analogue 6c.
Keywords: 3-Hydroxy group; Binding affinity; Molecular docking; Opioid receptors; Selectivity.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest
The authors declare no competing financial interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
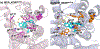
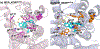
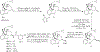
Similar articles
-
Structure selectivity relationship studies of 17-cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6β-[(4'-pyridyl)carboxamido]morphinan derivatives toward the development of the mu opioid receptor antagonists.Bioorg Med Chem Lett. 2011 Sep 15;21(18):5625-9. doi: 10.1016/j.bmcl.2011.06.135. Epub 2011 Jul 18. Bioorg Med Chem Lett. 2011. PMID: 21788135 Free PMC article.
-
Design, syntheses, and pharmacological characterization of 17-cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6α-(isoquinoline-3'-carboxamido)morphinan analogues as opioid receptor ligands.Bioorg Med Chem. 2015 Apr 15;23(8):1701-15. doi: 10.1016/j.bmc.2015.02.055. Epub 2015 Mar 6. Bioorg Med Chem. 2015. PMID: 25783191 Free PMC article.
-
Structure activity relationship studies of 17-cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6α-(isoquinoline-3'-carboxamido)morphinan (NAQ) analogues as potent opioid receptor ligands: preliminary results on the role of electronic characteristics for affinity and function.Bioorg Med Chem Lett. 2013 Sep 15;23(18):5045-8. doi: 10.1016/j.bmcl.2013.07.043. Epub 2013 Jul 31. Bioorg Med Chem Lett. 2013. PMID: 23948248 Free PMC article.
-
Rational drug design of selective epsilon opioid receptor agonist TAN-821 and antagonist TAN-1014.Curr Med Chem. 2006;13(10):1109-18. doi: 10.2174/092986706776360851. Curr Med Chem. 2006. PMID: 16719773 Review.
-
Morphinan derivatives--A review of the recent patent literature.IDrugs. 2003 Feb;6(2):129-37. IDrugs. 2003. PMID: 12789616 Review.
Cited by
-
Conformational Plasticity Enhances the Brain Penetration of a Metabolically Stable, Dual-Functional Opioid-Peptide CycloAnt.Int J Mol Sci. 2024 Oct 23;25(21):11389. doi: 10.3390/ijms252111389. Int J Mol Sci. 2024. PMID: 39518941 Free PMC article.
-
Characterization of a Potential KOR/DOR Dual Agonist with No Apparent Abuse Liability via a Complementary Structure-Activity Relationship Study on Nalfurafine Analogues.ACS Chem Neurosci. 2022 Dec 21;13(24):3608-3628. doi: 10.1021/acschemneuro.2c00526. Epub 2022 Nov 30. ACS Chem Neurosci. 2022. PMID: 36449691 Free PMC article.
-
Structural Characterization of KOR Inactive and Active States for 3D Pharmacology and Drug Discovery.Handb Exp Pharmacol. 2022;271:41-64. doi: 10.1007/164_2021_461. Handb Exp Pharmacol. 2022. PMID: 33945028 Review.
-
Blocking potential metabolic sites on NAT to improve its safety profile while retaining the pharmacological profile.Bioorg Chem. 2024 Jul;148:107489. doi: 10.1016/j.bioorg.2024.107489. Epub 2024 May 22. Bioorg Chem. 2024. PMID: 38797065 Free PMC article.
-
NCP, a Dual Kappa and Mu Opioid Receptor Agonist, Is a Potent Analgesic Against Inflammatory Pain without Reinforcing or Aversive Properties.J Pharmacol Exp Ther. 2024 Mar 15;389(1):106-117. doi: 10.1124/jpet.123.001870. J Pharmacol Exp Ther. 2024. PMID: 38409113 Free PMC article.
References
-
- Azzam AAH, McDonald J, Lambert DG, Hot topics in opioid pharmacology: mixed and biased opioids, Br. J. Anaesth 122(6) (2019) e136–e145. - PubMed
-
- Ma H, Obeng S, Wang H, Zheng Y, Li M, Jali AM, Stevens DL, Dewey WL, Selley DE, Zhang Y, Application of Bivalent Bioisostere Concept on Design and Discovery of Potent Opioid Receptor Modulators, J. Med. Chem 62(24) (2019) 11399–11415. - PubMed
-
- Yuan Y, Elbegdorj O, Chen J, Akubathini SK, Beletskaya IO, Selley DE, Zhang Y, Structure selectivity relationship studies of 17-cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-epoxy-6beta-[(4’-pyridyl)carboxa mido]morphinan derivatives toward the development of the mu opioid receptor antagonists, Bioorg. Med. Chem. Lett 21(18) (2011) 5625–9. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

