Serum cytokine levels of COVID-19 patients after 7 days of treatment with Favipiravir or Kaletra
- PMID: 33631512
- PMCID: PMC7826095
- DOI: 10.1016/j.intimp.2021.107407
Serum cytokine levels of COVID-19 patients after 7 days of treatment with Favipiravir or Kaletra
Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that causes coronavirus disease 2019 (COVID-19) has infected 86,4 M patients and resulted in 1,86 M deaths worldwide. Severe COVID-19 patients have elevated blood levels of interleukin-6 (IL-6), IL-1β, tumor necrosis factor (TNF)α, IL-8 and interferon (IFN)γ.
Objective: To investigate the effect of antiviral treatment serum cytokines in severe COVID-19 patients.
Methods: Blood was obtained from 29 patients (aged 32-79 yr) with laboratory-confirmed COVID-19 upon admission and 7 days after antiviral (Favipiravir or Lopinavir/Ritonavir) treatment. Patients also received standard supportive treatment in this retrospective observational study. Chest computed tomography (CT) scans were evaluated to investigate lung manifestations of COVID-19. Serum was also obtained and cytokines levels were evaluated. 19 age- and gender-matched healthy controls were studied.
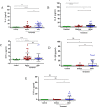
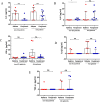
Results: Anti-viral therapy significantly reduced CT scan scores and the elevated serum levels of C-reactive protein (CRP) and lactate dehydrogenase (LDH). In contrast, serum levels of IL-6, IL-8 and IFNγ were elevated at baseline in COVID-19 subjects compared to healthy subjects with IL-6 (p = 0.006) and IL-8 (p = 0.011) levels being further elevated after antiviral therapy. IL-1β (p = 0.01) and TNFα (p = 0.069) levels were also enhanced after treatment but baseline levels were similar to those of healthy controls. These changes occurred irrespective of whether patients were admitted to the intensive care unit.
Conclusion: Antiviral treatments did not suppress the inflammatory phase of COVID-19 after 7 days treatment although CT, CRP and LDH suggest a decline in lung inflammation. There was limited evidence for a viral-mediated cytokine storm in these COVID-19 subjects.
Keywords: COVID-19; Cytokines storm; Flow cytometry; SARS-CoV-2.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
The comparison of favipiravir and lopinavir/ritonavir combination in COVID-19 treatment.Turk J Med Sci. 2021 Aug 30;51(4):1624-1630. doi: 10.3906/sag-2012-189. Turk J Med Sci. 2021. PMID: 33726482
-
Combination of (interferon beta-1b, lopinavir/ritonavir and ribavirin) versus favipiravir in hospitalized patients with non-critical COVID-19: A cohort study.PLoS One. 2021 Jun 10;16(6):e0252984. doi: 10.1371/journal.pone.0252984. eCollection 2021. PLoS One. 2021. PMID: 34111191 Free PMC article.
-
Nasopharyngeal SARS-CoV-2 Viral Load Response among COVID-19 Patients Receiving Favipiravir.Jpn J Infect Dis. 2021 Sep 22;74(5):416-420. doi: 10.7883/yoken.JJID.2020.827. Epub 2021 Jan 29. Jpn J Infect Dis. 2021. PMID: 33518623
-
Treatment options for COVID-19: The reality and challenges.J Microbiol Immunol Infect. 2020 Jun;53(3):436-443. doi: 10.1016/j.jmii.2020.03.034. Epub 2020 Apr 4. J Microbiol Immunol Infect. 2020. PMID: 32307245 Free PMC article. Review.
-
An overview of the safety, clinical application and antiviral research of the COVID-19 therapeutics.J Infect Public Health. 2020 Oct;13(10):1405-1414. doi: 10.1016/j.jiph.2020.07.004. Epub 2020 Jul 13. J Infect Public Health. 2020. PMID: 32684351 Free PMC article.
Cited by
-
Overview of clinical outcome and therapeutic effectiveness of Favipiravir in patients with COVID-19 admitted to intensive care unit, Riyadh, Saudi Arabia.J Infect Public Health. 2022 Apr;15(4):389-394. doi: 10.1016/j.jiph.2022.01.013. Epub 2022 Feb 15. J Infect Public Health. 2022. PMID: 35299062 Free PMC article.
-
A Pilot Study on Blood Components in COVID-19 Affected Subjects: A Correlation to UPR Signalling and ER-Stress.Indian J Clin Biochem. 2023 Jul;38(3):374-384. doi: 10.1007/s12291-023-01121-8. Epub 2023 Mar 9. Indian J Clin Biochem. 2023. PMID: 37223306 Free PMC article.
-
Effectiveness of Borage plus syrup on COVID-19 patients in intensive care units.Front Nutr. 2022 Nov 15;9:975937. doi: 10.3389/fnut.2022.975937. eCollection 2022. Front Nutr. 2022. PMID: 36458163 Free PMC article.
-
Programmed Cell Death Protein 1 (PD-1) Molecule in Coronavirus Disease 2019 (COVID-19)?Tanaffos. 2021 Jan;20(1):1-2. Tanaffos. 2021. PMID: 34394361 Free PMC article. No abstract available.
-
Th-1, Th-2, Th-9, Th-17, Th-22 type cytokine concentrations of critical COVID-19 patients after treatment with Remdesivir.Immunobiology. 2023 May;228(3):152378. doi: 10.1016/j.imbio.2023.152378. Epub 2023 Mar 24. Immunobiology. 2023. PMID: 37058846 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

