Co-overproducing Rubisco and Rubisco activase enhances photosynthesis in the optimal temperature range in rice
- PMID: 33631807
- PMCID: PMC8133551
- DOI: 10.1093/plphys/kiaa026
Co-overproducing Rubisco and Rubisco activase enhances photosynthesis in the optimal temperature range in rice
Abstract
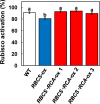
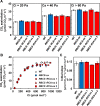
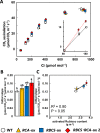
Rubisco limits C3 photosynthesis under some conditions and is therefore a potential target for improving photosynthetic efficiency. The overproduction of Rubisco is often accompanied by a decline in Rubisco activation, and the protein ratio of Rubisco activase (RCA) to Rubisco (RCA/Rubisco) greatly decreases in Rubisco-overproducing plants (RBCS-ox). Here, we produced transgenic rice (Oryza sativa) plants co-overproducing both Rubisco and RCA (RBCS-RCA-ox). Rubisco content in RBCS-RCA-ox plants increased by 23%-44%, and RCA/Rubisco levels were similar or higher than those of wild-type plants. However, although the activation state of Rubisco in RBCS-RCA-ox plants was enhanced, the rates of CO2 assimilation at 25°C in RBCS-RCA-ox plants did not differ from that of wild-type plants. Alternatively, at a moderately high temperature (optimal range of 32°C-36°C), the rates of CO2 assimilation in RBCS-ox and RBCS-RCA-ox plants were higher than in wild-type plants under conditions equal to or lower than current atmospheric CO2 levels. The activation state of Rubisco in RBCS-RCA-ox remained higher than that of RBCS-ox plants, and activated Rubisco content in RCA overproducing, RBCS-ox, RBCS-RCA-ox, and wild-type plants was highly correlated with the initial slope of CO2 assimilation against intercellular CO2 pressures (A:Ci) at 36°C. Thus, a simultaneous increase in Rubisco and RCA contents leads to enhanced photosynthesis within the optimal temperature range.
© American Society of Plant Biologists 2020. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures




References
-
- Bracher A, Whitney SM, Hartl FU, Hayer-Hartl M (2017) Biogenesis and metabolic maintenance of Rubisco. Annu Rev of Plant Biol 68: 29–60 - PubMed
-
- Busch FA, Sage RF (2017) The sensitivity of photosynthesis to O2 and CO2 concentration identifies strong Rubisco control above the thermal optimum. New Phytol 213: 1036–1051 - PubMed
-
- Carmo-Silva AE, Scales JC, Madgwick PJ, Parry MA (2015) Optimizing Rubisco and its regulation for greater resource use efficiency. Plant Cell Environ 38: 1817–1832 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

