Perspectives on improving light distribution and light use efficiency in crop canopies
- PMID: 33631812
- PMCID: PMC8133579
- DOI: 10.1093/plphys/kiaa006
Perspectives on improving light distribution and light use efficiency in crop canopies
Abstract
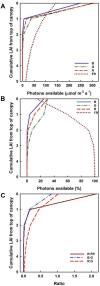
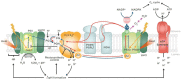
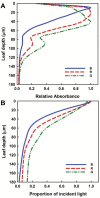
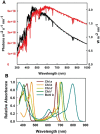
Plant stands in nature differ markedly from most seen in modern agriculture. In a dense mixed stand, plants must vie for resources, including light, for greater survival and fitness. Competitive advantages over surrounding plants improve fitness of the individual, thus maintaining the competitive traits in the gene pool. In contrast, monoculture crop production strives to increase output at the stand level and thus benefits from cooperation to increase yield of the community. In choosing plants with higher yields to propagate and grow for food, humans may have inadvertently selected the best competitors rather than the best cooperators. Here, we discuss how this selection for competitiveness has led to overinvestment in characteristics that increase light interception and, consequently, sub-optimal light use efficiency in crop fields that constrains yield improvement. Decades of crop canopy modeling research have provided potential strategies for improving light distribution in crop canopies, and we review the current progress of these strategies, including balancing light distribution through reducing pigment concentration. Based on recent research revealing red-shifted photosynthetic pigments in algae and photosynthetic bacteria, we also discuss potential strategies for optimizing light interception and use through introducing alternative pigment types in crops. These strategies for improving light distribution and expanding the wavelengths of light beyond those traditionally defined for photosynthesis in plant canopies may have large implications for improving crop yield and closing the yield gap.
© American Society of Plant Biologists 2021. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
Similar articles
-
Perspectives on improving photosynthesis to increase crop yield.Plant Cell. 2024 Oct 3;36(10):3944-3973. doi: 10.1093/plcell/koae132. Plant Cell. 2024. PMID: 38701340 Free PMC article. Review.
-
A canopy conundrum: can wind-induced movement help to increase crop productivity by relieving photosynthetic limitations?J Exp Bot. 2019 Apr 29;70(9):2371-2380. doi: 10.1093/jxb/ery424. J Exp Bot. 2019. PMID: 30481324
-
N uptake and distribution in crops: an agronomical and ecophysiological perspective.J Exp Bot. 2002 Apr;53(370):789-99. doi: 10.1093/jexbot/53.370.789. J Exp Bot. 2002. PMID: 11912222 Review.
-
Natural genetic variation in photosynthesis: an untapped resource to increase crop yield potential?Plant J. 2020 Feb;101(3):518-528. doi: 10.1111/tpj.14568. Epub 2019 Nov 13. Plant J. 2020. PMID: 31625637 Free PMC article. Review.
-
Can increased leaf photosynthesis be converted into higher crop mass production? A simulation study for rice using the crop model GECROS.J Exp Bot. 2017 Apr 1;68(9):2345-2360. doi: 10.1093/jxb/erx085. J Exp Bot. 2017. PMID: 28379522 Free PMC article.
Cited by
-
Yield of summer maize hybrids with different growth duration determined by light and temperature resource use efficiency from silking to physiological maturity stage.Front Plant Sci. 2022 Sep 29;13:992311. doi: 10.3389/fpls.2022.992311. eCollection 2022. Front Plant Sci. 2022. PMID: 36247586 Free PMC article.
-
Water-Light Interaction and Its Effect on the Morphophysiology of Cedrela fissilis Vell. Seedlings.Plants (Basel). 2024 Sep 22;13(18):2654. doi: 10.3390/plants13182654. Plants (Basel). 2024. PMID: 39339629 Free PMC article.
-
A missense mutation in the barley Xan-h gene encoding the Mg-chelatase subunit I leads to a viable pale green line with reduced daily transpiration rate.Plant Cell Rep. 2024 Sep 29;43(10):246. doi: 10.1007/s00299-024-03328-2. Plant Cell Rep. 2024. PMID: 39343835 Free PMC article.
-
Biology, Germination Ecology, and Shade Tolerance of Alkaliweed (Cressa truxillensis) and Its Response to Common Postemergence Herbicides.Plants (Basel). 2023 Jul 18;12(14):2679. doi: 10.3390/plants12142679. Plants (Basel). 2023. PMID: 37514293 Free PMC article.
-
Agriculture futurist: Don Ort.Plant Physiol. 2021 Feb 25;185(1):16-20. doi: 10.1093/plphys/kiaa024. Plant Physiol. 2021. PMID: 33631803 Free PMC article. No abstract available.
References
-
- Ainsworth EA, Davey PA, Bernacchi CJ, Dermody OC, Heaton EA, Moore DJ, Morgan PB, Naidu SL, Yoo Ra H-S, Zhu X-G, et al. (2002) A meta-analysis of elevated [CO2] effects on soybean (Glycine max) physiology, growth and yield. Glob Chang Biol 8: 695–709.
-
- Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165: 351–371. - PubMed
-
- Allen JF (1992) Protein phosphorylation in regulation of photosynthesis. Biochim Biophys Acta 1098: 275–335. - PubMed
-
- Allen JF, Bennett J, Steinback KE, Arntzen CJ (1981) Chloroplast protein phosphorylation couples plastoquinone redox state to distribution of excitation energy between photosystems. Nature 291: 25–29.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

